Breaking Up Is Hard to Understand
A compound dissolves when water molecules disrupt its chemical bonds and break it apart into ions. New research addresses a seemingly straightforward question: How many water molecules does this process require? The experiments suggest a tentative answer for hydrochloric acid ( ): between five and six. But the researchers also found an alternative interpretation of their data in which increasing the number of water molecules causes them to rearrange around the , rather than breaking it apart. Either way, they expect their new technique to help experimenters better understand the interactions of water with dissolving molecules.
To study the dissociation of molecules, researchers have observed their behavior in tiny droplets of just a handful of water molecules. There are important differences between such small clusters and a true aqueous environment, but the ability to perform detailed computer simulations of the clusters makes it possible to compare theory and experiment, says Vitaly Kresin of the University of Southern California in Los Angeles.
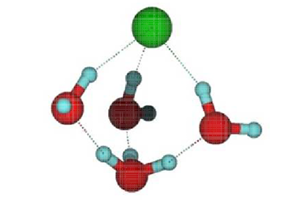
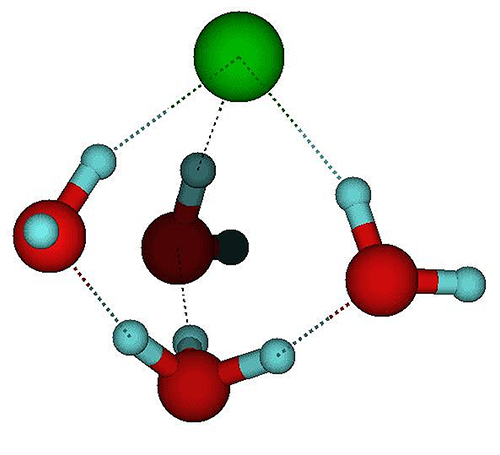
However, detecting when a molecule breaks apart is far from easy. Kresin and his colleagues came up with the idea that dissociation of a single molecule in a nanocluster would cause measurable changes in the cluster’s electric dipole moment. The dipole moment is a measure of the separation of positive and negative charges in an overall neutral object. The team reasoned that dissociation of the molecule would cause its ions to separate and water molecules (which have an intrinsic dipole moment) to rearrange themselves, leading to a redistribution of charge in the cluster as a whole. They chose to study , which has often been used as a test-case for the dissociation of acids in general.
The technique for measuring electric dipole moments of droplets came from earlier work by Kresin and other co-workers [1]. They create a stream of nanodroplets by sending water vapor through a tiny nozzle. The droplets then pass through a region of non-uniform electric field in which their deflections are a measure of their dipole moments. A movable slit selects a particular amount of deflection, and the droplets are then ionized by an electron beam to prepare them for a mass spectrometer (which requires charged particles). The mass spectrometer’s mass measurement provides the number of water molecules in each cluster.
Injecting into the water vapor would create a fraction of droplets containing a molecule of the acid. But Kresin had found in earlier experiments that ionizing such droplets knocked out the chlorine ion, making it impossible to tell which droplets had carried an acid molecule and which had not. So the team substituted deuterium, using DCl instead of . Even after the chlorine ion was ejected, droplets that had originally contained DCl would be slightly heavier than pure water droplets, a difference the mass spectrometer could detect. So the team could identify deuterium “doped” droplets and measure how their dipole moment depended on their size.
Looking at acid-containing droplets of three to nine water molecules, they found a distinct change in dipole moment when the number rose from five to six. This is consistent, Kresin says, with molecular simulations suggesting that about four water molecules should suffice to dissociate . But it’s also possible that a change in dipole moment could come about through a rearrangement of water molecules around an molecule that remained intact. So Kresin and his colleagues undertook some theoretical studies to sort through the possibilities.
When HCl dissociates in water, the free proton ( ) attaches itself to a water molecule, creating , but at modest temperatures the proton has enough energy to hop from one water molecule to another. In addition, ions have complex rotational and vibrational motions and can switch among these states by quantum tunneling. Kresin and his colleagues performed molecular simulations that examined the influence of these processes on the dipole moment.
The team concluded that it is unclear whether dissociation or reconfiguration of the nanoclusters was responsible for the measured change in electric dipole moment. Nevertheless, Francesco Paisani of the University of California at San Diego says that the work is significant for emphasizing the need to include both thermal and quantum effects in order to understand how experiments can probe cluster structure. It would be extremely interesting, he adds, to study how the results vary with temperature, which would offer further insight into the cluster chemistry.
This research is published in Physical Review Letters.
–David Lindley
David Lindley is a freelance science writer in Alexandria, Virginia.
References
- R. Moro, R. Rabinovitch, C. Xia, and V. V. Kresin, “Electric Dipole Moments of Water Clusters from a Beam Deflection Measurement,” Phys. Rev. Lett. 97, 123401 (2006)