Cloud Watchers
Researchers have demonstrated a new method of observing the electron “clouds” surrounding simple molecules. Using laser pulses, they split molecules of oxygen and nitrogen into pairs of ions, then reconstructed the shapes of the molecules’ original electron clouds, or “orbitals,” based on the ions’ paths. The results, published in the 10 September PRL, confirm the theoretical prediction that the likelihood of a molecule breaking up in an electric field depends on the shapes of its orbitals. The technique may help researchers probe reactions that occur in laser fusion systems, in the sun’s corona, and between biological molecules.
If molecules drift into a strong electromagnetic field, such as exists on the surface of the sun, they can’t help but ionize. Researchers can study this process in the lab using ultrashort laser pulses. The laser’s strong electric field forces a pair of electrons from a two-atom molecule, leaving two lone ions behind. Recent experiments suggested that molecules such as and tend to resist ionization compared with molecules such as –which was puzzling because the bonds that hold all three molecules together are about the same strength. The explanation seemed to be found in the orbitals various shapes: some orbital shapes align the electrons more readily with the laser’s electric field, which makes them more susceptible to ionization.
Lewis Cocke’s optical physics group at Kansas State University in Manhattan decided to test the idea with a new technique that the team believes is more direct than other methods of visualizing orbitals. They sprayed oxygen and nitrogen molecules across the path of a laser tuned to emit pulses eight femtoseconds long and 15 microjoules in energy–just enough to split a single molecule. A nearby detector plate collected the ions and recorded their positions. The researchers then reconstructed the orientation of the original molecule with respect to the laser’s electric field.
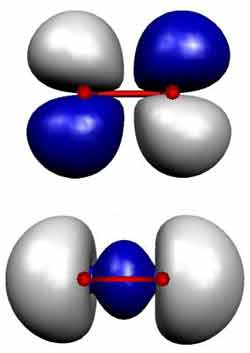
The team then constructed a map for each molecule. For each orientation angle they placed a point on the graph whose distance from the center corresponded to the probability of breaking up the molecule. Nitrogen, for example, ionized most easily when the laser’s electric field was aligned with the molecule’s long axis, and only rarely when the alignment differed. Nitrogen’s map looks like a long ellipse oriented horizontally, at zero degrees. That pattern matches the general shape of nitrogen’s outer orbital, known from separate calculations: it’s concentrated mainly along the line connecting the two atoms. Oxygen molecules, on the other hand, were more likely to break up when the electric field and molecular axis were offset by 45 degrees, corresponding to the four-leaf-clover shape of the molecule’s outer orbital.
The agreement between ionization data and the orbital shapes confirms recent theories on nitrogen and oxygen, says team member Ali Alnaser. He says the method could be used to study the orbitals and ionization of more complicated molecules, such as or He also envisions learning about the electronic structure of more complex biological molecules with more elaborate experiments.
“It is a beautiful illustration of the influence of molecular symmetry,” says chemist Henrik Stapelfeldt of the University of Aarhus in Denmark. “The resemblance of the measured angular distributions of the recoiling ions to the well known molecular orbitals of the valence electrons is striking.”
–JR Minkel
JR Minkel is a freelance science writer in New York City.


