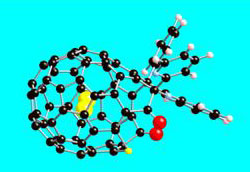
Molecule in a Cage
Objects exhibit quantum behavior when squeezed into a tight space. A new experiment has clearly demonstrated the wave-like properties of a hydrogen molecule inside a tiny carbon cage. Using neutrons to probe the state of the molecular prisoner, the researchers showed quantized states in both rotation and linear motion of the molecule, much like the “ladder” of excited electron states in an atom. The results, detailed in the 9 January Physical Review Letters, may help efforts to store hydrogen fuel for alternative-energy vehicles in similar carbon structures.
The quantum behavior of a particle becomes observable when it’s confined in a space nearly equal to its de Broglie wavelength, which depends on its momentum. For example, electrons confined to an atom have discrete (quantized) energy levels, unlike free electrons, and even molecules have discrete energy states associated with their motion when they are confined to a region that is small enough.
The hydrogen molecule ( ) is a natural choice for studies of quantum dynamics, due to its small mass and the unique quantum restrictions on its rotational states. Researchers have seen quantized motion of hydrogen molecules inside porous materials [1] and on soccer-ball-shaped molecules called buckyballs [2]. But either the systems have been too complicated or the measuring devices have not had sufficient resolution to obtain a clear picture of the quantization. Now Anthony Horsewill of the University of Nottingham in England and his colleagues have devised a textbook example of fully quantized molecular motion where the energy levels are distinct. They used a recently-developed technique [3] in which chemical reactions create openings in buckyballs. The holes allow single hydrogen molecules to enter each of the balls, and after filling the cages, the team cooled them to 2.5 degrees Kelvin and exposed them to a beam of neutrons.
The neutrons either slowed down (lost energy) or sped up (gained energy) as a result of interactions with the trapped hydrogen molecules. A plot of the neutron energy spectrum showed several peaks in the range between 10 and 20 milli-electron-volts, which corresponds to transitions between energy levels in the hydrogen molecule. These aren’t electronic transitions, but discrete jumps in the energy of the entire molecule.
Some of the energy jumps were between rotational states of the hydrogen. Rotational states are quantized even for uncaged molecules, but for hydrogen they are strongly tied to the spins of the two nuclei, thanks to a quantum effect involving the Pauli exclusion principle. This relationship typically inhibits transitions between the rotational ground state (called parahydrogen, in which the nuclear spins are oppositely directed) and the first rotational excited state (called orthohydrogen, where the spins are aligned). However, neutrons are capable of flipping one of the spins, and thereby inducing rotations in the molecule. By varying the temperature of their sample, the team was able to verify which peaks in the spectrum corresponded to these rotational transitions.
The remaining peaks were identified as “translational” (linear motion) transitions. As a hydrogen molecule rattles around inside its cage, its quantum wave takes on standing wave patterns similar to the modes of a vibrating violin string. “This is a beautiful example of the wave-like nature of massive particles,” Horsewill says. The team showed that the wavelength of the trapped hydrogen–inferred from the momentum transfer of the neutrons–was consistent with previous estimates of the diameter of the cage interior (1.56 Ångstroms).
Buckyballs and their carbon nanotube cousins have been considered as storage mediums for hydrogen fuel, so future work on these hydrogen-carbon interactions may provide useful insights, Horsewill says. But for now, the experiment appears to stand on it own. “It presents some important information on a very fundamental issue, the translational-rotational behavior of the simplest molecule, in a very constrained environment,” says chemist Nick Turro of Columbia University. Stephen FitzGerald of Oberlin College, who has studied similar molecule-buckyball combinations, says this simple system will be a great teaching example: “It allows me to once more say, ‘hey, this quantum mechanics stuff really works.’”
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics Magazine based in Lyon, France.
References
- L. Ulivi et al., “Quantum rattling of molecular hydrogen in clathrate hydrate nanocavities,” Phys. Rev. B 76, 161401 (2007)
- S. A. Fitzgerald et al., “Quantum dynamics of interstitial H2 in solid C60,” Phys. Rev. B 60, 6439 (1999)
- K. Komatsu, M. Murata, and Y. Murata, “Encapsulation of Molecular Hydrogen in Fullerene C60 by Organic Synthesis,” Science 307, 238 (2005)
More Information
Focus story: Nuclei Affect the Bounce of H2
Focus story: Buckyballs inside Nanotubes
Wikipedia: Spin Isomers of Hydrogen
Periodic table of atoms trapped in buckyballs (a.k.a. fullerenes)