Ripples on a Cell
Detailed new observations of waves rippling across the surfaces of living cells may give researchers new insight into how such waves are formed. A team publishing in the 4 December Physical Review Letters used a sensitive microscopy technique to track surface ripples propagating backward from the edges of human skin cells that have the ability to move into the region of a wound. The results suggest that elements of the cell’s movement machinery are responsible for the waves.
Several research groups have observed waves called membrane ruffles in cells that are capable of migration. A migrating cell moves like an amoeba, extending one or more feelers, which adhere to their surroundings and then contract to pull the rest of the cell along. As the feeler contracts, waves ripple backward across its top surface. The biochemical details of cell movement are hard to sort out, but some researchers suspect the ruffles are a by-product of events in the cytoskeleton, a network of proteins that gives the cell shape and directs its movements.
The flexible cell membrane is held up like a tent by a rigid mesh of long, polymer filaments called actin. Protrusion of a feeler starts when proteins that promote polymerization of actin–the creation of filaments–gather at the cell edge, where they generate extra actin mesh, which bows the membrane outward. Eventually, myosin proteins, which connect the actin filaments in the mesh, begin to slide the filaments past one another in a way that causes a large-scale contraction–a pulling-in force that draws the rest of the cell in the direction of the bulging membrane.
In a recent model [1] researchers proposed that the waves on the top surface of a cell’s extended feelers are essentially smaller versions of migration events: polymerization promoters generate a wave of actin filament creation at the edge of the feeler, which triggers a wave of myosin retraction after a short delay. The myosin contraction pulls down on the trailing edge of the wave, which introduces extra curvature in the membrane at the leading edge. The model proposed that the extra curvature attracts more polymerization promoters, which renews the cycle and leads to a propagating wave. The model predicts specific frequencies and amplitudes for the waves, but no one has been able to measure them on living cells very precisely.
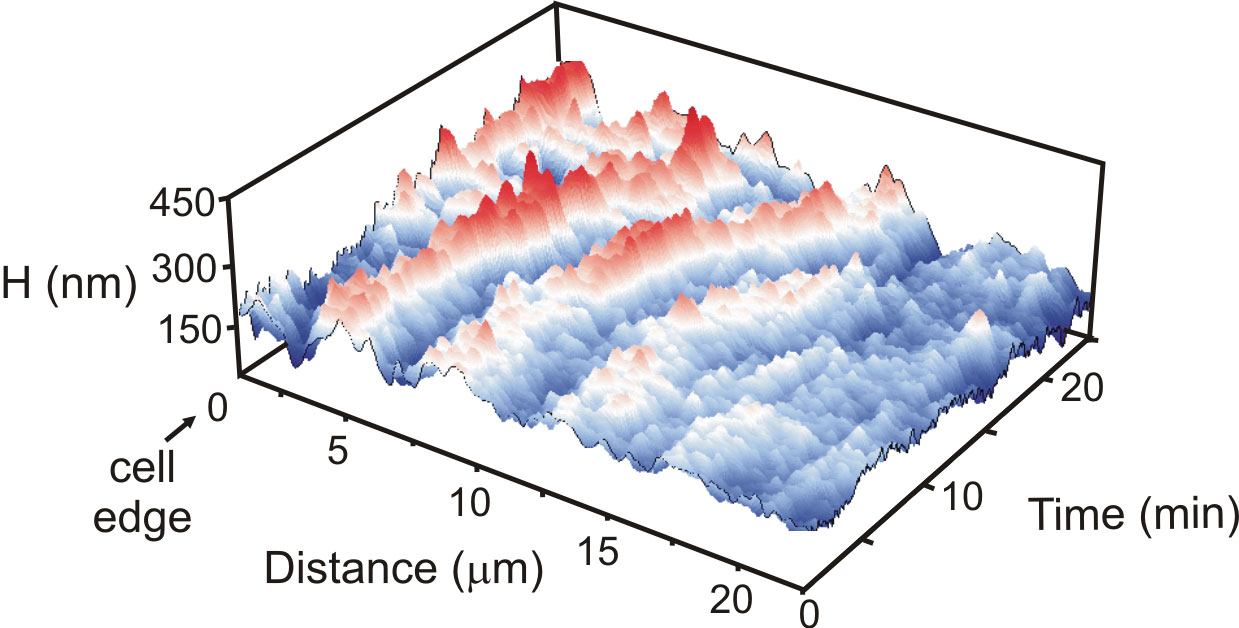
To see if they could resolve these wave features, Chau-Hwang Lee and his colleagues from the Academia Sinica and the National Yang-Ming University in Taiwan used a microscopy technique they developed for resolving nanoscale features on the cell surface. Called non-interferometric wide-field optical profilometry (NIWOP), it involves recording the light reflected by the sample when it is placed slightly out of focus. Small variations in height show up as small variations in the intensity of reflected light.
The team measured propagating waves up to 300 nanometers high on 23 human fibroblast cells, skin cells that migrate to fill in wounds. According to the model, wave speed depends on the concentration of myosin, which Lee says is higher toward the center of the cell than at the front edge of a feeler. Accordingly, the researchers observed that the waves accelerate from 10 nanometers per second near the edge to a maximum of about 25 nanometers per second after traveling 20 microns. The waves also shrank as they propagated, as would be expected if actin polymerization proteins became less concentrated away from the cell edge. The team also observed the predicted relationships among wavelength, frequency, and amplitude of the waves.
The wave measurements “seem consistent and convincing,” says Oscar Mesquita of the University of Minas Gerais in Brazil. He says prior microscopy studies haven’t provided such detail and that the new work shows that the simple elements of the model suffice to explain a complicated behavior. Lee says it remains to be seen whether the same model suffices to explain membrane ripples in other cell types. His group’s next step will be to test what happens to the waves when different components of the cytoskeleton are stimulated or switched off.
–JR Minkel
JR Minkel is a freelance science writer in New York City.
References
- R. Shlomovitz and N. S. Gov, Membrane Waves Driven by Actin and Myosin, Phys. Rev. Lett. 98, 168103 (2007)