How Thin Films Melt
If you heat a crystal that is just a few molecules thick, it melts differently from a macroscopically thick crystal. A team using gel-like beads in liquid as stand-ins for molecules examined melting in this “thin film” regime by tracking the motion of each particle. As they report in the 21 May Physical Review Letters, films thicker than four layers melt by passing through a phase that is part liquid and part solid, whereas films of two to four layers melt all at once. Single-layer films pass through a completely different phase between solid and liquid, they found.
Researchers would like to know how phase transitions, such as from solid to liquid, change when the substance is confined to fewer dimensions than the usual three. They have fairly good theories on the melting of a single layer of molecules, but more recently they have pursued the thin film regime–a few molecular layers. Thin films have properties in common with both 2D and 3D, and their melting is not well understood. For experiments, it’s difficult to track individual molecules, so researchers often turn to microscopic plastic spheres floating in liquid, which act somewhat like molecules and can be tracked individually with a microscope and video processing software.
Researchers have studied the phase behavior of spheres packed into an enclosure. But it’s been difficult to observe melting, says Yilong Han of the Hong Kong University of Science and Technology, because even when the spheres are heated up, they don’t have a lot of room to move. Han and his colleagues demonstrated in 2008 that spheres made of a polymer called N-isopropylacrylamide (NIPA) could avoid this problem [1]. When heated from 24 to 28 degrees Celsius, the spheres decrease their volume by about 27 percent. The extra space allows the particles to move away from their lattice sites, like molecules in a liquid, and the team observed single-layer melting.
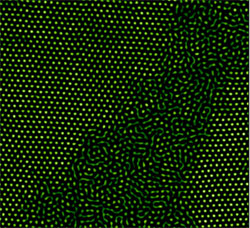
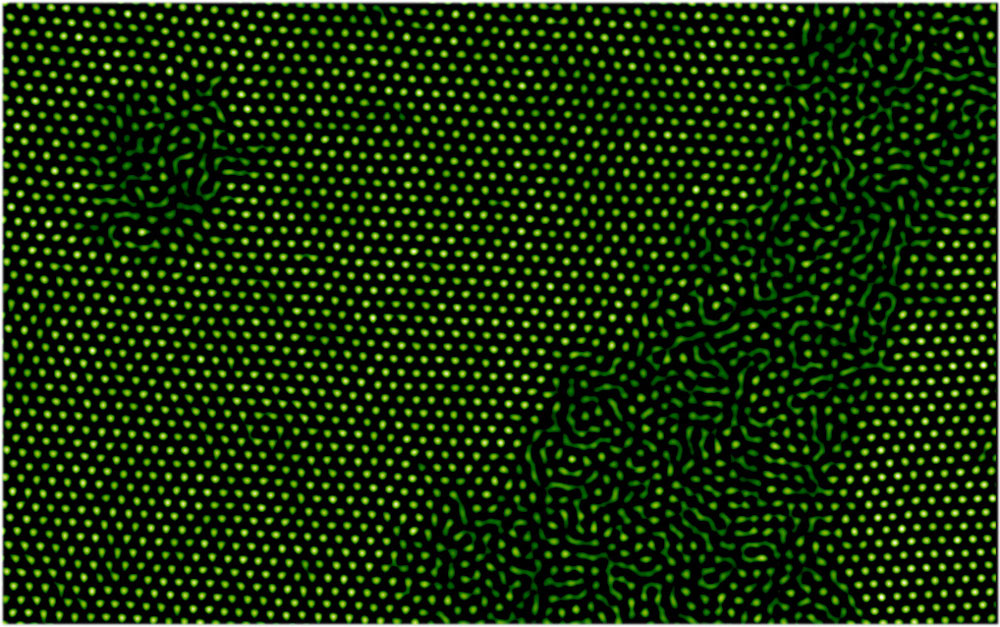
In their latest work, Han and his colleagues explored thicker samples, to look for the differences among single-layer, thin-film, and “bulk” behavior of melting microspheres. The researchers packed fluorescently labeled NIPA spheres between glass slides and then raised the temperature. They used a confocal microscope to track every particle in 3D. For films thicker than four layers, the spheres became disordered in pockets that grew from the sphere-glass boundary or from boundaries between differently-oriented crystalline regions. This phase is the liquid-solid coexistence regime, known from macroscopic samples to exist over a narrow range of temperatures around the melting point. Eventually the disordered regions grew until the whole film had become disordered, or “liquid.”
For films between two and four layers thick, melting occurred all at one time across the whole film. For single-layer films, melting followed predictions of the standard 2D theory and again occurred in two steps. The spheres first adopted the “hexatic” configuration, a 2D liquid phase that maintains an orderly six-fold symmetry even as the particles move around. Eventually the hexatic pattern broke down into the random disorder of a conventional liquid. Han says a detailed explanation of the more sudden melting of thin films will take more work on both theory and experiments, but it does show similarities with previous observations of 2D melting.
“To me it’s fascinating that a five-layer thick crystal is already thick enough to melt in the same way as a big 3D crystal sample,” says Eric Weeks of Emory University. “It says that you have to be really, really thin in order to change the physics of crystal melting.” It makes sense that the “netherworld” between 2D and 3D would show mysterious behavior, says David Nelson of Harvard University. Not only are thin films subject to thermal fluctuations, but they also involve packing geometries that wouldn’t normally occur in bulk samples. “A complete theory might have to take into account all these fascinating physical features,” he says.
–JR Minkel
JR Minkel is a freelance science writer in New York City.
References
- Y. Han, N. Y. Ha, A. M. Alsayed, and A. G. Yodh, “Melting of Two-Dimensional Tunable-Diameter Colloidal Crystals,” Phys. Rev. E 77, 040406 (2008)