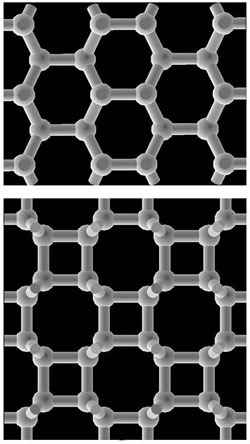
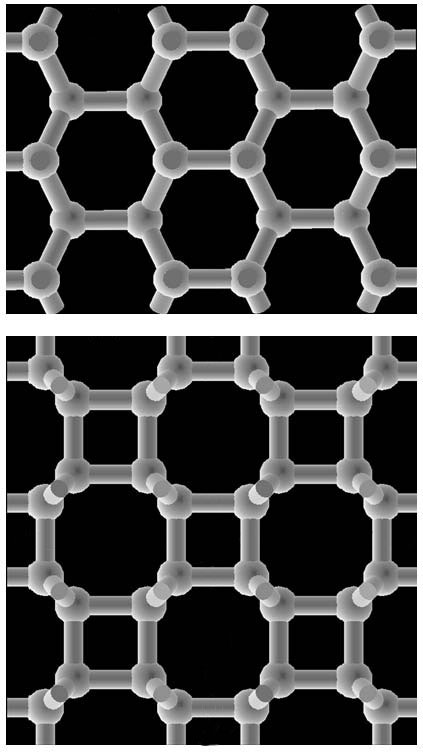
Between Graphite and Diamond
Pure carbon has a wide variety of atomic structures, such as diamond and graphite, but the structure created by a 2003 graphite compression experiment has been controversial. Now two teams of theorists have followed different lines of evidence to suggest that the structure involves a 3D network of four-atom rings called bct-carbon. In the 26 March Physical Review Letters, one team reported simulations that agree with the 2003 data, and in the October Physical Review B, the other team shows that the energy barrier to create bct-carbon is low enough that it could have appeared in the 2003 experiment. Researchers say the results point to the existence of a new and unexpectedly simple form of carbon.
Since the early 1960s researchers have known that graphite undergoes a reversible transformation when subjected to high pressure at room temperature, known as cold compression. But the chemical and structural changes to the carbon atoms remained largely mysterious. Then in 2003 a team reported cold compression of graphite in a diamond anvil press and simultaneous exposure of the sample to x rays to study its chemical bonding [1]. At pressures greater than 17 gigapascals (170,000 atmospheres), the sample entered a phase in which the carbon atoms formed three or four chemical bonds with their neighbors. The structure was distinct from diamond, which appears at higher temperatures and pressures. “It looked closely related to graphite,” says Wendy Mao of Stanford University, who was part of the 2003 study, but it could scratch diamond.
Since then, researchers have been trying to come up with the three-dimensional structure of cold compressed graphite. In principle, when individual sheets of graphite (known as graphene) are pressed together, the layers should buckle, allowing chemical bonds to form between adjacent layers. Researchers in 2009 proposed that cold compressed graphite consisted of a mixture of two forms of carbon. The first was graphite, which has six-membered rings arranged in sheets, and the second was a relatively new structure called M-carbon, in which the layers link up to form five- and seven-membered rings when viewed edge-on [2].
A group of American and Japanese researchers, including Takashi Miyake of Japan’s National Institute of Advanced Industrial Science and Technology and Renata Wentzcovitch of the University of Minnesota, felt this result might not be the whole story. In earlier simulations by members of the group, atoms of carbon nanotubes under pressure partially rearranged themselves into four-membered rings [3] which had been previously postulated for other situations. So the team used quantum mechanical simulations to analyze so-called bct-carbon (“body-centered tetragonal”), which has four-membered rings connected in a three-dimensional network. They reported in PRL in March that bct-carbon is more stable than graphite at a pressure of 18.6 gigapascals. The team also simulated the x-ray diffraction pattern of M-carbon mixed with a small amount of bct-carbon and found that this combination closely matched the x-ray diffraction pattern reported in 2003.
Bct-carbon is also implicated in the latest installment to the cold compressed graphite story. A group led by Hui-Tian Wang of Nankai and Nanjing universities in China came at the problem in a different way. Wang and his colleagues simulated the change of state from graphite to each of three different forms: diamond, M-carbon, and bct-carbon. The team simulated a series of intermediate states for each final structure, calculating the total energy of the system at each step. According to their results, bct-carbon has the lowest energy barrier when forming from graphite at high pressure, suggesting that the diamond-anvil experiment could easily have created it. But the team doesn’t rule out the possibility of M-carbon appearing as well.
Artem Oganov of Stony Brook University in New York agrees that both M-carbon and bct-carbon may be involved in cold compressed graphite. He is working with colleagues on simulations in which the change of structure starts at a small number of nucleation centers, rather than happening everywhere at once. This favors M-carbon, he says, but these simulations don’t account for the single direction of pressure applied in the 2003 experiment, which could allow some bct-carbon. It would be fitting if both forms appeared, he says. “They are both so beautiful and so simple that I think both of them should be synthesizable.”
–JR Minkel
JR Minkel is a freelance science writer in New York City.
References
- W. L. Mao et al., “Bonding Changes in Compressed Superhard Graphite,” Science 302, 425 (2003)
- Q. Li et al., “Superhard Monoclinic Polymorph of Carbon,” Phys. Rev. Lett. 102, 175506 (2009)
- Y. Omata et al., “Nanotube Nanoscience: A Molecular-Dynamics Study,” Physica E (Amsterdam) 29, 454 (2005)