The Skeleton Dance
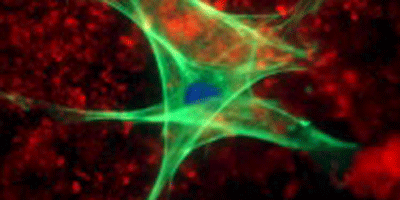
In vital processes such as wound healing or embryonic growth, cells must move and change their shapes in a complex choreography. To make this happen, the cell’s skeleton (cytoskeleton) provides the cell with rigidity and shape, while various motor proteins play the role of muscles connecting to, and moving along, cytoskeletal components. Researchers know that adenosine triphosphate (ATP) fuels the mechanical activity of these molecular motors, but the microscopic mechanisms by which the motors direct the cytoskeleton have yet to be elucidated.
To shed light on such mechanisms, Björn Stuhrmann, of the FOM Institute for Atomic and Molecular Physics in the Netherlands, and colleagues investigated a simplified model system in vitro, studying how myosin, a motor protein involved in many cellular motility processes, drives structural rearrangements in a network of actin filaments, one of the main components of the cytoskeleton. As reported in Physical Review E, the authors initiated the polymerization of actin filaments into a network, in the presence of active myosin motors, which were activated by a sudden jump in the ATP concentration. They then monitored the ensuing dynamics by video-tracking the motion of tracer beads that were embedded in the actin network.
Stuhrmann et al. identified which motions were directly driven by myosin and which arose instead from random fluctuations. This enabled them to show that, in the initial stages, myosin motors exert tensile forces that cause the filament network to contract, but after minutes the active motors can no longer rearrange the network and a stationary state is reached. The ability to see few motor-directed displacements in a bath of stochastic fluctuations will be crucial for gaining a deeper microscopic understanding of motor-driven cellular processes. – Matteo Rini