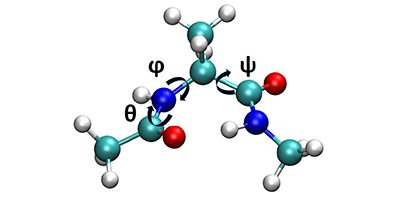
Filling Holes in Molecular Dynamics
Molecular-dynamics simulations have been used for decades to study the dynamics of molecules and materials. Yet for all the computer power now available, these simulations can, at best, capture only about one millisecond of molecular motion, while many phenomena would require tracking longer time spans. Pratyush Tiwary and Michele Parrinello of the University of Lugano (USI) in Switzerland, report in Physical Review Letters a possible way around this so-called “time-scale” problem.
The time-scale bottleneck is a consequence of the way molecular systems evolve—moments of frantic activity separated by long spells in stable free energy wells or metastable states. In simulations, tracking the dynamics of the system with atomic resolution requires extremely fine femtosecond time steps, but those small steps add up enormously to the computational complexity during the quieter stretches when not much is going on, but the system is still evolving.
Among many attempts to get around this problem, a technique called metadynamics (developed earlier by Alessandro Laio and Parrinello) keeps track of the history of the computation and prevents it from getting repeatedly stuck in free energy wells already visited. This is analogous to gradually filling the known energy wells with computational sand to help move the system along. However, whereas only static information about the free energy surface could previously be obtained from metadynamics, the new work by Tiwary and Parrinello allows them to estimate actual transition rates.
Tiwary and Parrinello applied their new approach to a few model systems used in previous molecular dynamics tests. They find excellent agreement between their results and long unbiased molecular-dynamics simulations, and a speed-up over the latter of three to five orders of magnitude, thereby reaching well beyond the millisecond timescale. – David Voss