Terahertz-Driven Chemistry
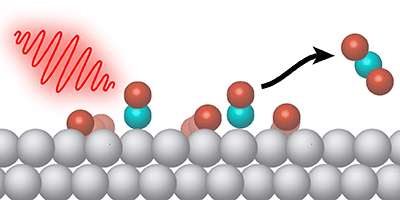
Certain chemical reactions occur at a higher rate when the reactants are stuck to a two-dimensional surface. But sometimes the desired reaction competes with other processes. To control the surface chemistry, Jerry LaRue, from the SLAC National Accelerator Laboratory in California, and his colleagues demonstrate a new technique in which pulses of light in the terahertz frequency range selectively drive an oxidation reaction on a metal surface. The pulses effectively gives a kick to adsorbed oxygen atoms, so that they move along the surface and interact with other molecules more often.
The surface reaction studied by LaRue et al. is the conversion of carbon monoxide ( ) and oxygen into carbon dioxide ( ). This oxidation process is commonly performed in a car’s catalytic converter, where metals serve as the surface catalyst. The researchers looked specifically at ruthenium—a relatively inexpensive catalyst material but one in which CO oxidation must compete with CO desorbing from the surface.
Previous work showed that optical pulses could increase oxidation on ruthenium, but the pulses also heat the surface, causing increased desorption. In their experiments, LaRue and his colleagues utilized terahertz pulses produced at SLAC’s Linac Coherent Light Source. The team showed that the electric field from the pulses causes electrons to oscillate near the surface, and this electron motion weakens the bonds that hold oxygen atoms to the metal. The partly released oxygen atoms have extra energy for moving along the surface and colliding with molecules forming . The terahertz pulses do not increase the surface temperature, so the desorption rate remains unchanged. The researchers found that terahertz stimulation led to as much as a third of adsorbed converting to .
This research is published in Physical Review Letters.
–Michael Schirber