Stop moving!
A molecule is an object that moves, rotates, and vibrates. We have known, at least in principle, since the first papers on quantum physics, how to properly describe molecular rotations and vibrations, and this in turn strongly supported the development of quantum mechanics in the early days. It was generally accepted that molecules in a gas bottle occupy their energy levels statistically, typically determined by the bottle temperature, and that this is what can be studied spectroscopically. In recent years this has changed significantly and the precise control of molecular properties has come sharply into focus. Today, many groups work on the preparation of large ensembles of molecules in pure quantum states that can also be controlled coherently with electromagnetic fields. Writing in Physical Review Letters, Xin Tong, Alexander Whinney, and Stefan Willitsch of the University of Basel, Switzerland, now report a powerful new way to gain control over a wide range of molecular ion species [1].
But why are controlled molecular ensembles interesting at all? Don’t we know everything we need to know about molecules? It may be typical of molecules that the topics that drive the interest in this field are quite diverse. The energy levels of well-controlled molecules will allow studies with extreme spectroscopic resolution, where not only quantum mechanics and its application to coupled systems of many atoms is tested to unprecedented levels, but energy shifts due to parity violation, a finite value of the electron electric dipole moment, or a variation of fundamental constants, such as the proton-to-electron mass ratio, should come within reach. We have also known for a long time that molecular collision and reaction cross sections can strongly depend upon the vibrational and rotational quantum state. Consequently, quantum-state controlled molecules can teach us new insights into how chemical reactions occur. And then molecules that are prepared in a single long-lived quantum state can be coupled with electromagnetic fields to a second long-lived state and thereby realize well-controlled ensembles of two-level systems. Such ensembles are exciting because they offer more options than atoms in the representation of qubits for quantum information processing.
The diversity of interests from fundamental physics all the way to chemical reaction dynamics makes it necessary to develop general methods to prepare molecules in single quantum states. To make the situation for experiments even more complicated, most of the above mentioned applications also require the molecules to be trapped at low temperatures. Therefore efficient cooling schemes for the translational motion of the molecules are also needed. Tong et al. demonstrate a versatile new method to achieve all of this for molecular ions in a radio frequency trap. They form nitrogen dimer ions in the rotational and vibrational ground state, embedded into the Coulomb crystal of laser-cooled calcium ions [1].
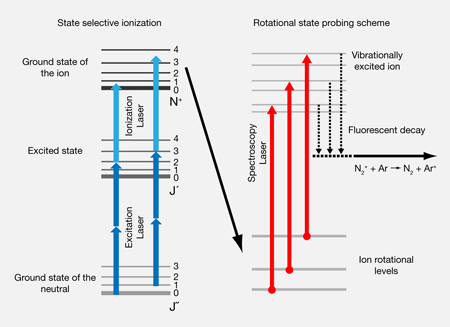
Previous methods to bring molecular ions to the vibrational ground state use ion traps in which the ions are kept long enough to thermalize with an inert buffer gas [2]. Alternatively, spontaneous infrared emission radiatively cools trapped molecular ions and brings them to the vibrational ground state [3]. Both methods cool vibrational motion well within one second, provided the temperature of the buffer gas or the ambient blackbody radiation, respectively, is much lower than the splitting between the ground and first excited vibrational level. Since this temperature is currently technologically limited to be near that of liquid helium ( or about ), vibrational cooling alone can actually be achieved for many stable molecules, as typical vibrational energy level splittings range between and . Rotational energy levels, however, are much more closely spaced [Fig. 1(a)], so that even at liquid helium temperature most molecules feature a statistical population of many rotational states. For example, for the nitrogen dimer cation studied by Tong et al., only half of the population would be found in the ground rotational state at .
Ultracold, subkelvin, internal temperatures therefore have to be approached differently. Very recently, two research groups achieved rotational cooling of trapped molecular ions using elaborate schemes to pump the ions into the ground rotation state, which combined laser excitation and radiative emission with blackbody-induced rethermalization [4]. Tong et al. have now managed to prepare a homonuclear molecular ion, the nitrogen dimer cation, in its lowest rovibrational quantum state. In their experiment they used laser-ionization of nitrogen molecules to form ions, with the laser frequency carefully adjusted to couple exactly one rovibrational quantum state in the neutral molecule with another one in the ion. Careful play with the laser frequency and the optical selection rules allowed Tong et al. to not only prepare the ground rotational state but also select excited rotational states in this way [Fig. 1(b)]. This resonantly enhanced multiphoton ionization process was driven with the laser and the molecular beam crossed in the center of a radio frequency Paul trap. Already filled with laser-cooled calcium ions, this trap also captured the nitrogen molecular ions. Then the Coulomb interaction between the calcium and the nitrogen ions sympathetically cooled the latter to millikelvin temperatures and incorporated them into a mixed-species Coulomb crystal.
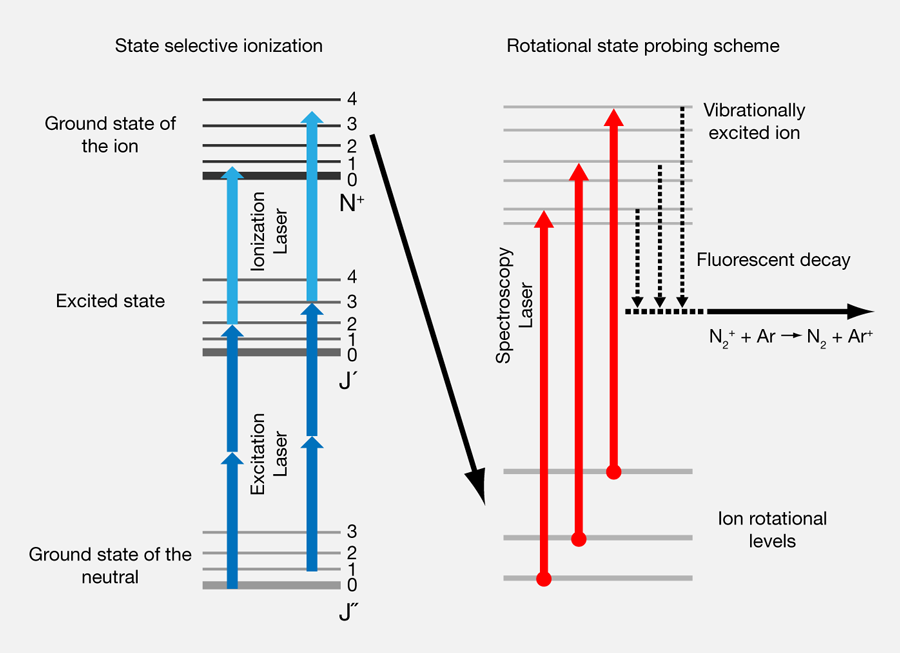
Of course this preparatory step was only the first part of the experiment. For the second part, the measurement of the rotational level population of the trapped ions, again a combination of experimental tricks was needed. The rotational states were addressed with a single-photon transition to the first excited electronic transition in the ion [Fig. 1(c)]. In a detection scheme termed “chemical probing,” the optical excitation induced a chemical reaction with argon gas added to the background gas of the ion trap. If the addressed rotational state of was populated, the absorption then led to the formation of by charge transfer with the laser-excited ions. This change of ion species inside the Coulomb crystal was observed in the fluorescence images of the ions in the Coulomb crystal. In contrast to the calcium ions, the and ions do not fluoresce themselves, but they can be distinguished because the argon ions occupy random positions in the crystal, whereas the lighter nitrogen ions are always located near the symmetry axis. In this clever, indirect way, Tong et al. find of the ions in the rotational ground state, and, specifically, they do not find any evidence for excited rotational states. Alternatively, Tong et al. showed that they can also prepare the molecular ions in a selected excited rotational state and thereby create population inversion.
For all practical purposes the ion does not couple to the electromagnetic field of the blackbody radiation because its transition dipole moment vanishes due to the inversion symmetry. Thus straight heating by blackbody radiation does not occur in such molecules. This also allowed Tong et al. to observe collisional changes of the rotational population of the trapped ions over the time scale of several minutes. In particular, they observed the decay of an excited rotational state—a demonstration of the long-term stability of the prepared internal states in the trap.
With the work of Tong et al., the experimental techniques for the control of molecules in all possible degrees of freedom has been enriched by a very interesting new method. Resonantly enhanced multiphoton ionization is already applied to many molecules to measure rotational state populations of the neutral form. Its extension to the state-control of molecular ions will allow many different species to be prepared in the low-temperature environment of a laser-cooled Coulomb crystal. Which of these will be a successful system for molecular ion quantum information or for novel ultracold chemistry? The future will tell.
References
- X. Tong, A. H. Winney, and S. Willitsch, Phys. Rev. Lett. 105, 143001 (2010)
- D. Gerlich, Phys. Scr. T59, 256 (1995)
- Z. Amitay et al., Science 281, 75 (1998)
- P. F. Staanum, K. Højbjerre, P. S. Skyt, A. K. Hansen, and M. Drewsen, Nature Phys. 6, 271 (2010); T. Schneider, B. Roth, H. Duncker, I. Ernsting, and S. Schiller, 6, 275 (2010)