New Molecular Probe Uses Gold Antennas
The antenna inside your smart phone efficiently picks up the electromagnetic transmissions used by the device. Borrowing this trick, researchers have used tiny gold antennas to boost the power of infrared techniques that record the twists and turns of chemical bonds. The method could track the workings of cell membranes and help chemists develop responsive surface coatings that react to changing conditions.
Chemical bonds typically vibrate, stretch, or wiggle when they absorb specific wavelengths of infrared (IR) light, making IR spectroscopy a versatile tool for molecular studies. In a recently developed technique known as nonlinear vibrational spectroscopy, an ultrashort pulse of IR light, lasting tens of femtoseconds or less, sets a bond in motion. Then a second pulse hits the bond a few picoseconds later. Because the bond is now moving in some way, it absorbs the light differently than it did the first time. By hitting a bond with a series of ultrashort pulses and detecting the transmitted light, researchers can create snapshots of the bond’s ultrafast motion. Such methods have been used to observe the formation and breaking of hydrogen bonds, the unfolding of proteins when exposed to heat, and the disassociation of ion pairs in water.
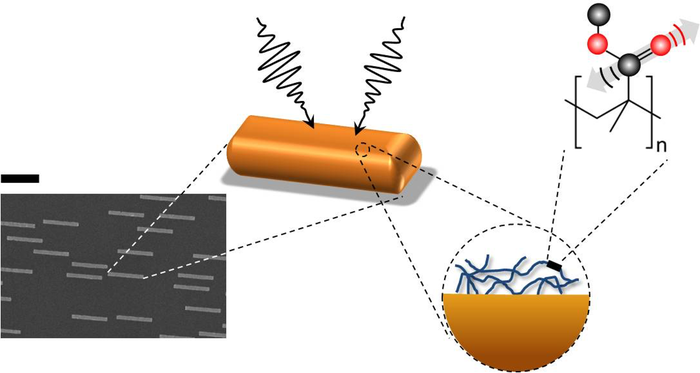
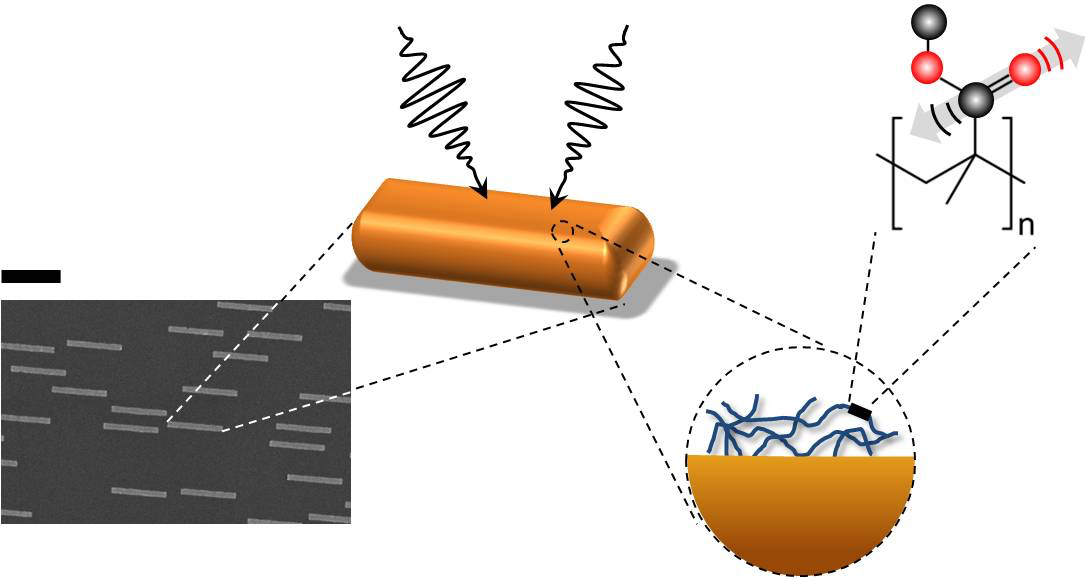
In theory, this method could also be useful in studying thin molecular surfaces, such as the lipid bilayer of a cell membrane. But there’s a problem: most molecular bonds are inefficient at capturing IR radiation. Although a bond’s vibration frequencies correspond to those of IR light, its size—less than a nanometer—is much smaller than micrometer-scale IR wavelengths. This mismatch makes chemical bonds poor antennas for IR radiation, and small biological samples do not contain enough molecules to generate a detectable response for nonlinear vibrational spectroscopy to work. To get around this difficulty, Yves Rezus and his colleagues at the FOM Institute for Atomic and Molecular Physics (AMOLF) in Amsterdam decided to provide some help in the form of tiny gold antennas.
Rezus and his colleagues fabricated gold strips, nanometers (nm) wide, nm thick, and nm long, on a transparent base. Preliminary experiments showed that strips with these dimensions are good antennas for -nm IR light, a wavelength the researchers selected because it excites carbon-oxygen (C-O) bonds. They then deposited on these antennas a -nm-thick layer of polymethylmethacrylate (PMMA), a transparent plastic also known as Lucite or Plexiglas that contains a high density of C-O bonds.
Using a pump-probe technique in which one laser pulse excites the sample and a second tests how the sample changes in response, the researchers found that the PMMA plus gold antenna arrangement yielded a signal that was ten thousand times greater than the PMMA alone. “We went from something that was barely detectable to—Bam!—this huge signal,” says Rezus.
This enhancement occurs because of “near-field” electromagnetic effects close to the antennas. The gold strips absorb and re-radiate IR radiation, but at distances of more than a few wavelengths, the reradiated waves are similar to the incoming radiation and no better at exciting C-O bonds. However, each PMMA film is within a few nanometers of an antenna, where the electric and magnetic fields take on a complex form that more efficiently excites C-O bonds in the PMMA.
Although other researchers have explored near-field effects of small antennas, this is the first application to nonlinear vibrational spectroscopy. The team believes that by fine-tuning the shape of the antennas they could boost the sensitivity of the technique by another factor of 10, making it useful for investigating biological materials that have fewer C-O bonds than PMMA. Rezus says the technique could probe how drugs permeate through cell membranes or capture the inner workings of so-called responsive surfaces—materials that alter their color in the presence of a chemical or repel dirt or odors.
Javier Aizpurua of the Materials Physics Center in San Sebastián, Spain, who studies metallic nanoantennas and ultrafast phenomena, says the method is “a very nice new thing that opens the door to addressing the dynamics of very low amounts of molecules.”
This research is published in Physical Review Letters.
–Adam Mann
Adam Mann is a freelance science writer in Oakland, California.