A New Theory of Etching
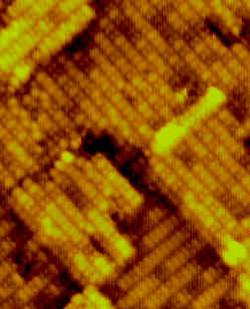
Practiced as an art form since at least the Middle Ages, chemical etching is now an essential part of producing silicon microchips. But this process of removing atoms from a silicon surface is not well understood at the atomic level. Now three scientists report in the 18 January PRL that they have used a scanning tunneling microscope (STM) to verify a new model for the sequence of atomic motions that contribute to etching. They found that increasing the etchant beyond an optimal value actually reduces the etching, a counterintuitive prediction of the model.
The ancients used acids to etch surfaces, but chip manufacturers use gases containing halogen atoms, giving rise to the term “dry etching.” On the silicon (100) surface–the crystal face studied by the PRL authors–neighboring silicon atoms lower their energy by moving slightly together to form chains of dimers, like dancers lined up two-by-two. Halogen atoms, such as chlorine, can stick to the surface by forming bonds with the silicon atoms in the dimers. To begin the dynamic dance of dry etching, a halogen atom hops from one silicon atom in a dimer to the other, leaving one silicon with two halogens and its partner with none. Heating the silicon to several hundred degrees Celsius facilitates the hopping and causes the volatile silicon dihalide to leave the surface, or “desorb.” The partner left behind then moves on top of the layer of paired silicon atoms.
This simple picture implies that the etching rate should increase with the concentration of halogen, but now John Weaver of the University of Minnesota in Minneapolis and his colleagues have shown otherwise. Last year theorists calculated that a silicon atom with two halogens is on average more likely to return one of them to its partner than to desorb (which requires both halogens). They suggested that the halogen return could be eliminated if the halogen-free partner moved away quickly enough. In this scheme, too much halogen actually slows down the etching, because the partner cannot leave if nearby surface bonds are already tied up by halogens.
Weaver and his colleagues made atomic-scale STM images of silicon before and after etching with successively higher chlorine concentrations. They demonstrated that the etching rate reached a maximum and then decreased with increasing chlorine, in quantitative agreement with the predictions of the silicon-vacancy model. “This study demonstrates quite elegantly the dynamical nature of the etching process,” says John Boland of the University of North Carolina. He points out that the silicon-vacancy pathway is just one of several etching mechanisms, and the new results suggest that these mechanisms may be controllable, to achieve specific etching patterns.
–Arthur Robinson
Arthur Robinson is a science writer and editor.


