Proteins as Shock Absorbers
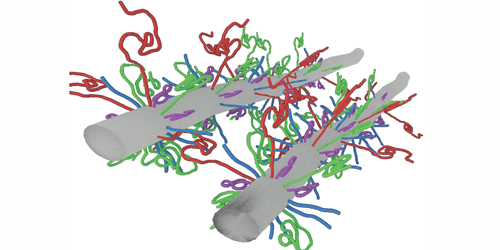
Neurofilament proteins form a filamentous network that mechanically supports nerve cells, and they are critical for structural integrity and proper neuronal function. These proteins are often exposed to large compression stress, resulting, for example, from the transport of cellular organelles through the network. But it is not clear how neurofilament proteins can withstand such stresses. Now, Micha Kornreich and co-workers from Roy Beck’s team at Tel Aviv University, Israel, have experimentally measured and theoretically modeled the compression response of neurofilament proteins, showing that these proteins function like shock absorbers.
The authors focused on three different types of neurofilament proteins derived from bovine spinal cord. These proteins consisted of bottlebrush-shaped filaments that differed in their charge distribution and the length of their “tails”—long unfolded segments of molecules that protruded from the main filament backbone. The researchers compressed the filamentous network and resolved its structure using a small-angle x-ray scattering technique. Their results revealed that the network’s mechanical and structural properties were governed by the tails’ molecular composition and the surrounding environment. Under low mechanical stress, the properties of the network were dictated by specific attractive interactions between the tails. In contrast, when the proteins experienced large stresses, they condensed to form an intertwined network of filaments whose mechanical response to compression was analogous to that of a nonideal gas. Accordingly, Beck and his colleagues modeled the interactions between the tails using a single fitting parameter akin to a spring constant and showed that the proteins themselves remained intact, even after the network contracted by 20-fold in volume.
This research is published in Physical Review Letters.
–Katherine Kornei
Katherine Kornei is a freelance writer based in Portland, Oregon.