Have Water, Will Charge
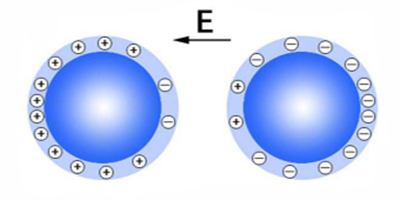
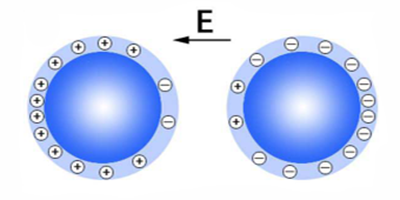
Two identical insulators, despite having no free electrons, are able to charge one another upon contact. This process, called “contact electrification,” is important both in natural phenomena (like lightning near volcanic dust plumes) and in industrial applications (like laser printing). However, the physical explanation for this effect has remained incomplete: the identity of the charge carriers and what drives their movement are unknown in many cases. Now, experiments conducted by Yonghong Liu’s group at the China University of Petroleum (East China) have suggested that, in humid environments with strong electric fields (e.g., thunderstorms), the charge carriers are likely hydronium ( ) and hydroxide ( ) ions, driven by the electric field along water adsorbed on the surfaces of the insulators.
Liu and his collaborators tested how insulating glass spheres obtained net charges in the presence of an electric field and under various humidity conditions. The results suggest that contact electrification requires the glass beads to have adsorbed water from a humid atmosphere and that hydronium and hydroxide ions, derived from the dissociation of water molecules, segregate on the spheres’ surfaces in the presence of an electric field and serve as charge carriers. The researchers propose that when two spheres collide, the ions that come into contact neutralize each other within the water bridge that connects the insulators, leaving each sphere with a net charge (the ions on each sphere’s opposite side). Although the water films on the sphere surfaces are likely not uniform, the authors propose, based on previous experiments by other groups, that the ions can still be transferred between isolated water islands through surrounding water vapor connecting the islands.
This research is published in Physical Review X.
–Katherine Kornei