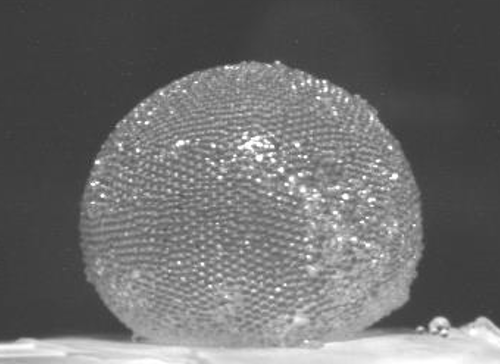
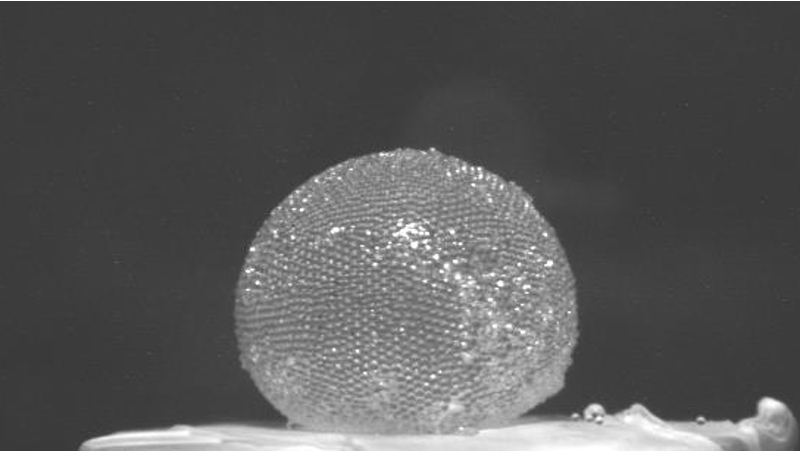
“Gas Marbles” Store Air in Strong Spheres
French researchers have discovered how to wrap up a gas in a kind of bubble that is much stronger than the sort you can blow from a soap film. They call their bubbles gas marbles. The spheres have a tough shell made from a closely packed layer of tiny plastic particles, and the team says that the marbles could be useful for a wide range of purposes, such as stabilizing foams or sequestering toxic gases.
Gas marbles are relatives of so-called liquid marbles—droplets of liquid coated with microscopic, liquid-repelling beads, which can roll around on a solid surface without breaking apart [1]. Liquid marbles might find uses as chemical sensors or microreactors. They maintain their shape because of the surface tension and incompressibility of the liquid, but gas marbles have only vapor inside.
Another similar structure called an armored bubble has also been studied previously [2]. These gas bubbles within a liquid have their gas-liquid interface strengthened by the absorption of water-repelling particles.
Gas marbles were discovered in 2015 when then-graduate-student Yousra Timounay of the Université Paris-Est (UPE) was studying a film of plastic (polystyrene) microspheres floating on the surface of a solution similar to soapy water. She submerged a rectangular wire frame that was roughly a centimeter on each side, just as one dips a wire loop into a soap solution to blow bubbles.
Timounay found that if the frame was wide enough and the microspheres were at least several hundred micrometers in diameter, then lifting the frame generated a gas marble. During lifting, the film on the frame would spontaneously fold in on itself and detach to form a gas marble that would drop to the liquid surface below. “The full mechanism of gas-marble creation is not fully understood for the moment, but their creation is reproducible,” says Timounay’s Ph.D. advisor Florence Rouyer.
Timounay, Rouyer, and their UPE colleague Olivier Pitois showed that the “bubbles” consist of a spherical shell of wet microspheres packed closely together. Neighboring spheres are bridged by a liquid meniscus that “glues” them together via surface tension. A typical gas marble is several millimeters across, trapping perhaps several hundred cubic millimeters of air inside.
Although the trapped air is at atmospheric pressure, the researchers could alter the internal pressure using a syringe that punctures the “skin” without rupturing it. They found that the relative pressure could be increased or decreased by more than 10 times without damaging the gas marble, but eventually, at extreme pressures, it burst or collapsed. Unlike soap bubbles, gas marbles don’t change their size during inflation or deflation because of the strength of the microsphere-and-liquid skin.
The structures are robust enough to hold and roll in your hand, Rouyer says, as long as your hands are clean, and you don’t press too hard. They behave more or less like elastic, spherical shells, except that gas marbles can be reconstituted after they break apart. The team hopes that this strength and ability to reform will be useful. For example, gas marbles could be used to stabilize foams—armored bubbles have been used to that end previously [3]—and to store helpful gases or incarcerate dangerous ones.
The usefulness of these objects for gas storage will depend on how easily gas diffuses through the skin (the permeability). Rouyer says that the team will be reporting permeability measurements soon, but they estimate that it should be very low.
“These gas marbles are a new and fascinating concept,” says chemical engineer Valeria Garbin of Imperial College London. However, mechanical engineer Howard Stone of Princeton University in New Jersey warns that the structures may fall apart once the liquid in their shells evaporates.
Vincent Craig of the Australian National University in Canberra says that “like much elegant work, some might claim these results are ‘obvious’—but only in retrospect.” Craig speculates about other potential uses based on gas trapping. For example, gas marbles could deliver radio-tracer gases in the lungs for medical imaging or deliver gaseous odors on demand. “‘Scratch and sniff’ as the bubbles are crushed,” he says.
This research is published in Physical Review Letters.
–Philip Ball
Philip Ball is a freelance science writer in London. His latest book is How Life Works (Picador, 2024).
References
- P. Aussillous and D. Quéré, “Liquid Marbles,” Nature 411, 924 (2001).
- M. Abkarian, A. Subramaniam, S. Kim, R. Larsen, S. Yang, and H. Stone, “Dissolution Arrest and Stability of Particle-Covered Bubbles,” Phys. Rev. Lett. 99, 188301 (2007).
- B. Binks and T. Horozov, “Aqueous Foams Stabilized Solely by Silica Nanoparticles,” Angew. Chem., Int. Ed. 44, 3722 (2005).