A Physical Model for Neurodegenerative Disease
In a number of neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and ALS (amyotrophic lateral sclerosis), malformed proteins spread throughout the brain, triggering cell damage and death. A new model analyzes how these molecules diffuse through the brain’s highly nonuniform structure and shows that the distribution of harmful proteins in each disease depends on where the proteins first appear. The model also suggests that brain damage results primarily from the body’s removal of brain tissue damaged by the physical impact of these proteins. The researchers hope the new model will be a useful tool in efforts to impede disease progression.
Brain scans show that Alzheimer’s disease is marked by fibrous tangles consisting of misfolded forms of a protein known as amyloid- 𝛽. A distorted form of another protein, TDP-43, is linked to ALS and certain forms of dementia. In these and other cases, the origin of the malformed proteins remains murky, but once present, they act as templates that cause normal copies of these proteins to misfold in the same way. Scientists believe that disease progression occurs as the harmful proteins spread through the brain and aggregate in fibers and clumps.
Researchers have considered cellular and biochemical mechanisms that cause the harmful proteins to spread and multiply. But Alain Goriely of the University of Oxford in the UK and his colleagues constructed a model that ignores biochemistry and depicts the two basic processes of propagation and increasing numbers in elementary ways.
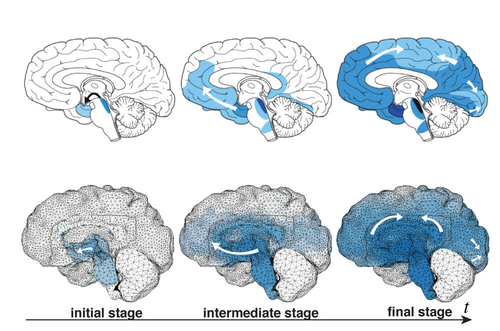
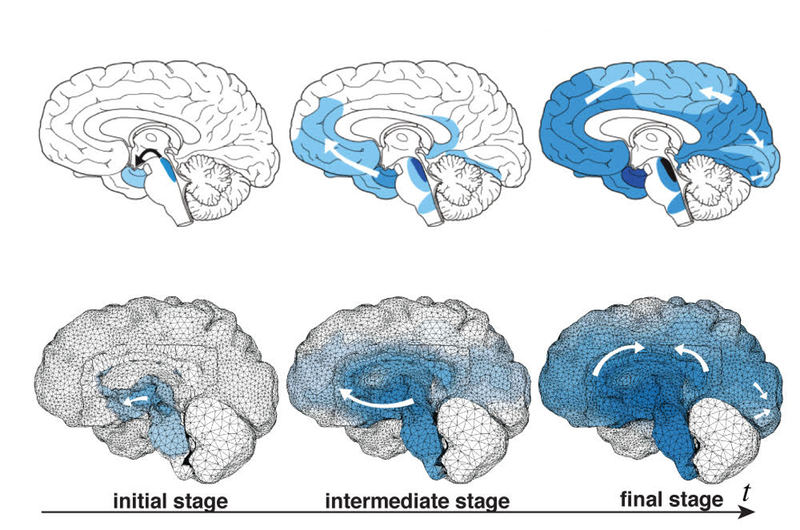
The harmful proteins are known to travel preferentially through the network of nerve cells (neurons) in the brain, rather than through the fluid outside of cells. Each nerve cell has a small cell body that contains its nucleus and a long extension called an axon that transmits electrical signals to other neurons. Axons constitute the bulk of the brain’s white matter, while their associated cell bodies comprise most of the gray matter. In the team’s model, harmful proteins move through the white matter by diffusing along axons at a certain rate while diffusing out of axons and into extracellular space at a much lower rate. In the gray matter, the harmful proteins are assumed to diffuse isotropically at an intermediate rate. To mimic the formation of additional malformed proteins, the model assumes that once the bad version of a protein diffuses into a new region, it triggers the appearance of copies of itself at a rate that depends only on the number of harmful proteins present.
Goriely and his colleagues used MRI images of the brain of a 32-year-old man to construct high-resolution computer simulations of the brain’s structure in both two and three dimensions. The 3D simulations included the whole brain; the 2D simulations were carried out in vertical slices, either front-to-back or side-to-side. To “seed” their simulations, the researchers drew on clinical data mapping the progress of Alzheimer’s and Parkinson’s diseases and ALS. Brain scans show that the harmful proteins tend to show up first at certain brain locations and then spread in a characteristic pattern. The researchers started their simulations with initial conditions drawn from such scans and were surprised at the accuracy with which their straightforward model reproduced the patterns associated with each disease.
To estimate the damage to brain tissue, the team made another simple assumption. They assumed that as harmful proteins aggregate in the gray matter and reach critical concentrations, their physical presence exerts pressure on the soft neuronal tissue to the point that the body’s repair mechanisms begin to target and remove damaged cells. As with the spread of misfolded proteins, the pattern of atrophy in the simulations was a good match to the pattern of brain damage seen in patients with the corresponding diseases, which are marked by loss of brain function tied to the atrophied areas.
Kristian Franze, who studies neuronal mechanics at the University of Cambridge, says the research is a “great example of how we can learn about a biological system by considering [its] physical aspects.” He adds that the movement of harmful proteins along axons will undoubtedly depend on biochemical mechanisms but says the new model is “very valuable” in showing that on the large scale, simple physical laws govern how such proteins spread. Henning Voss, a radiologist at Weill Cornell Medical School in New York City, agrees that the model shows that diffusion is an important physical aspect of neurodegenerative disease progression. He says the new results open “a nice playing field for future interdisciplinary research.”
This research is published in Physical Review Letters.
–David Lindley
David Lindley is a freelance science writer, now retired. His most recent book is The Dream Universe: How Fundamental Physics Lost Its Way (Penguin Random House, 2020).