Video—Growing a Crystal One Atomic Layer at a Time
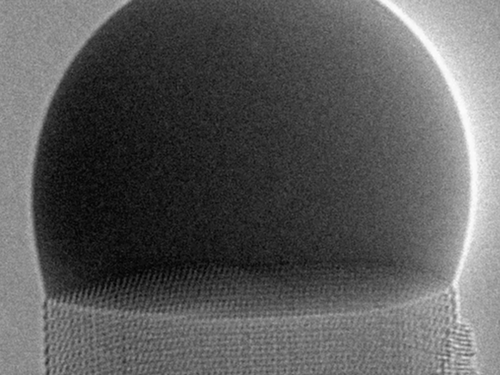
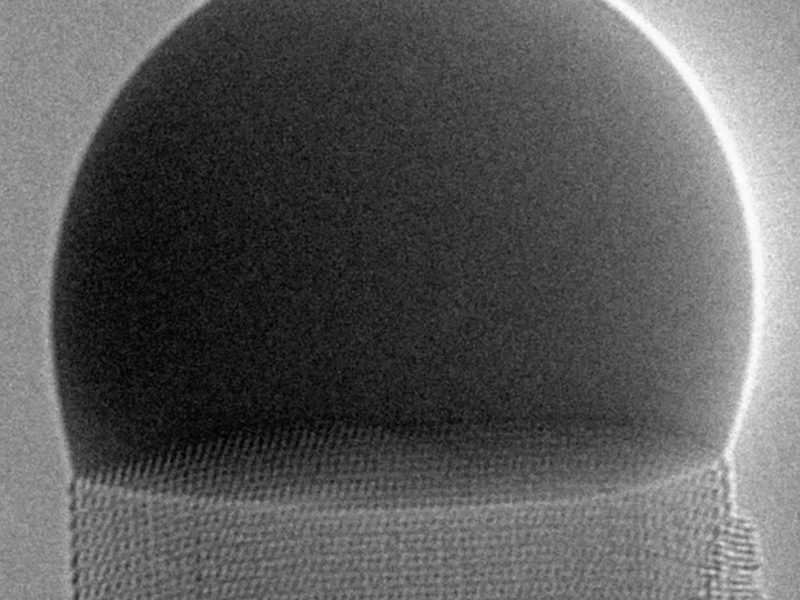
A crystal grows as each atom falls into its place in an ordered structure, and now a research team has imaged the process with atomic detail for a nanocrystal growing up underneath a liquid droplet. Each new atomic layer of the crystal begins at one corner of the hexagonal top surface, forming an atomic step that spreads across the surface and lifts the droplet by a tiny amount. Explaining the progression of the atomic steps with a simple theory led to insights that could help researchers better understand the crystallization process in other situations.
In the video, produced by Jean-Christophe Harmand and Gilles Patriarche of the French National Center for Scientific Research (CNRS) and the University of Paris-Saclay and their colleagues, the droplet above the crystal is made of liquid gold that is supersaturated with gallium and arsenic. The saturation causes the two elements to drop out of solution and form a gallium arsenide nanocrystal, which the team continuously images using a transmission electron microscope. As seen from above, the front edge of the expanding step changes from convex to concave as it moves across the surface. The researchers developed a theory that explains this shape evolution, showing the influence of the energy of the advancing step and of the boundaries on the process of crystal growth.
This research is published in Physical Review Letters.
–David Ehrenstein
David Ehrenstein is a Senior Editor for Physics Magazine.