Rinsing is Key to Removing Stains
Getting out a tough stain requires applying detergent and then rinsing with fresh water. The rinse cycle helps flush away loosened dirt and other muck, but it may also help to pull out particles stuck inside narrow pores in the fabric. This secondary mechanism—based on dirt being drawn from high to low concentrations of detergent—had gone unnoticed, but new microfluidic experiments show that concentration-based effects can be highly effective in removing deep-down dirt. The results could help in developing new detergents, or designing an optimal wash-rinse cycle, and may have implications in other areas, such as enhanced oil recovery.
Detergents and other cleaning products contain surfactants, which are molecules that improve the mixing between water and other substances, such as oil or grease. The surfactant allows water to penetrate into a stain and lift the dirt particles off the fabric; the rinse then sweeps the dirt away. However, many fabrics contain small “dead-end” spaces that seem immune to rinsing. For example, water can pass over the threads in a cotton T-shirt, but flow is restricted in between the tightly wound fibers that make up each thread. Yet packed-in dirt somehow manages to escape from these small spaces. This so-called “stagnant core mystery” is well known in the laundry detergent industry, says Sangwoo Shin from the University of Hawaii at Manoa.
To resolve this mystery, Shin and his colleagues explored the role played by diffusiophoresis, the migration of a particle in the direction of increasing or decreasing concentration of some molecule (called the solute) in the fluid. The solute could be a salt ion or other charged molecule, in which case any variation (gradient) in its concentration will produce an electric field that can drive particle migration. The process superficially resembles chemotaxis, in which bacteria move toward increasing nutrient concentrations, says Shin.
Shin and co-workers previously studied diffusiophoresis as a way to insert particles (such as drugs or disinfectants) into dead-end pores [1]. Cleaning a fabric means running that process in reverse. Most detergents use ionic surfactants, so one can expect that a gradient in the surfactant will generate an electric field. However, surfactants have an additional property: they will normally attach to a particle in the solution. Thus a neutral particle can become charged when detergent molecules attach to it.
To explore diffusiophoresis in fabric cleaning, Shin and his colleagues ran experiments in a microfluidic channel connected to a series of 50-micrometer-wide, dead-end pores. When fluid flowed through the main channel, there was little flow into the pores, mimicking the situation of stagnant cores in fabrics. The team allowed dirt—represented by micrometer-sized, fluorescent, polystyrene particles—to accumulate inside the pores. They then mixed in a detergent, sodium dodecyl sulfate, which produces a negatively charged surfactant ion in solution.
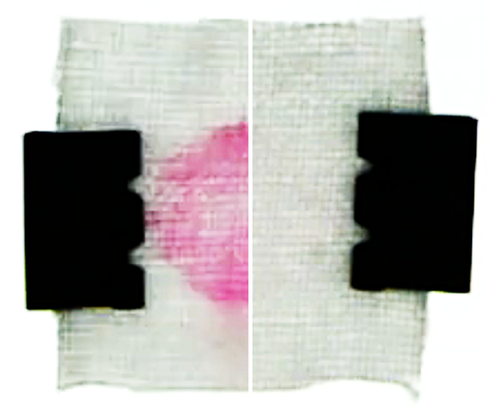
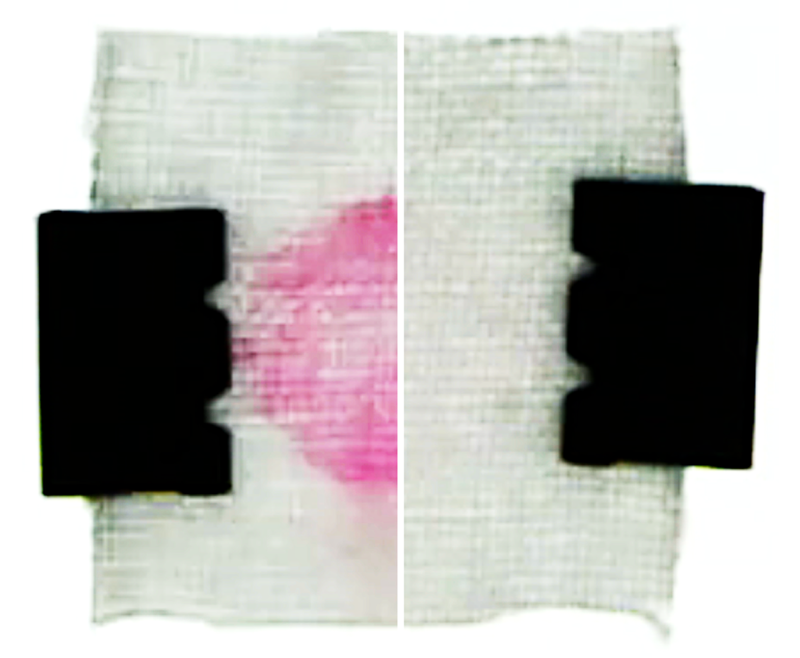
As a control test, the team flushed the channel with detergent-filled water. This flow was unable to dislodge particles in the pores, and as the concentration remained more or less uniform, there was no help from diffusiophoresis. That changed when the team rinsed with fresh water. The rinse flushed away most of the detergent, except deep in the pores where the surfactant ions remained concentrated. The resulting solute gradient created an electric field that helped to draw surfactant-covered particles out of the pores. Under the influence of this diffusiophoresis, the pores nearly emptied out after 10 minutes of rinsing (see first video).
The team also conducted model laundry experiments. The results showed that, after soaking a stained cotton fabric with detergent, a fresh water rinse cleans faster and more thoroughly than a rinse with detergent-filled water (see second video). Based on these findings, the researchers make several recommendations for optimizing the cleaning process. One of them is that fresh water should be added rapidly after thoroughly wringing out fabrics to remove detergent-saturated water.
Soft-matter physicist Jérémie Palacci from the University of California, San Diego, says the experiment is very elegant, and the effect of diffusiophoresis is unexpectedly strong. He wonders, though, why some stains (like coffee) seem more stubborn than others. Chemical engineer Stefano Guido from the University of Naples Federico II in Italy says that the results could have widespread benefits. “These findings could be exploited to develop more effective detergents and might have an application in other fields, such as enhanced oil recovery by water flooding.”
This research is published in Physical Review Applied.
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics Magazine based in Lyon, France.
References
- S. Shin, E. Um, B. Sabass, J. Ault, M. Rahimi, P. Warren, and H. Stone, “Size-Dependent Control of Colloid Transport via Solute Gradients in Dead-End Channels,” Proc. Natl. Acad. Sci. U.S.A. 113, No. 2 (2015).