A Prediction for “Hot” Superconductivity
Since the 1911 discovery of superconductivity in mercury, the phenomenon has been observed in more than 30,000 elements, alloys, and compounds. And yet the number of devices hosting superconductors is still limited. The main reason is that these materials transition to the superconducting state at temperatures well below room temperature. The tide may be changing, however, with the recent discoveries of high transition temperatures ( ’s) in hydrogen-rich materials called hydrides. Now, Yanming Ma at Jilin University, China, and collaborators predict that the hydride compound, , should have a of around 473 K— —but only when the material is subjected to a pressure of 250 GPa [1]. This level of pressure is on par with that of Earth’s core, so their idea would be difficult to test in the lab. But the result is exciting because it is the highest predicted for any material, and it unveils a potentially new pathway to achieving superconductivity at room temperature.
Condensed-matter physicists have devoted enormous theoretical, computational, and experimental efforts to finding a room-temperature superconductor. The work has led to the discoveries of many types of high- materials, including the copper- and iron-based family of compounds, fullerides, and magnesium diboride. In all of these cases, the materials were discovered in the lab before theorists tried to understand them.
Hydrides have been a game changer in this trend, with theory jump starting experiment. These compounds comprise hydrogen and one or more elements that easily give up electrons. Interest in these materials as a route to high began with a 1971 paper by John Gilman and was resuscitated by Neil Ashcroft in 2004 [2]. The attractive feature of hydrides is their closeness to metallic hydrogen, which has two key ingredients for superconductivity: high phonon frequencies and strong electron-phonon coupling. When compressed, a hydride is similar to metallic hydrogen, thus opening the door to superconductivity. Usually the required pressures for metallization are extremely high, but researchers have found ways to lower this pressure below the predicted metallization threshold for solid hydrogen. One mechanism is to introduce impurities that modulate the hydride’s structural and electrical properties. Another way is through chemical “precompression,” where chemical elements are added into the hydrogen lattice.
Initially, predicting which hydrides would have a crystal structure favoring superconductivity proved challenging. But in 2014, Ma and colleagues showed that the hydride would stay structurally intact up to high pressure, where high- superconductivity was expected [3]. This simulation led to the experimental discovery of superconductivity at 203 K in the related compound under a pressure of 150 GPa [4, 5].
A further step came in 2017, when Ma and collaborators and Russell Hemley and his collaborators predicted a new hydride, , which was expected to have higher than that of [6]. In contrast with , has a higher concentration of hydrogen atoms, which form a cage-like structure that holds the lanthanum ions. This “host-guest” structure is thought to favor superconductivity. Moreover, its hydrogen content is much higher than in and closer to that of solid hydrogen. Motivated by the prediction, Hemley’s group and an independent team led by Mikhail Eremets studied the material at pressures of 120 to 185 GPa. Both found evidence that is a superconductor with a between 250 and 260 K—about 40 K below room temperature [7]. These are the highest recorded ’s of any superconductor, and the discoveries have been a major step towards room-temperature superconductivity.
So far, most of the hydride predictions have focused on two-element (binary) compounds [5]. The new paper by Ma and co-workers moves into the mostly uncharted territory of three-element (ternary) hydrides [1]. Vastly more compounds are possible by expanding to ternaries. But predictions also become harder because there are more structural configurations to consider for three elements versus two. Also, the number of ways that a candidate structure can decompose is much greater. For this reason, relatively little is known about the phase stability and configurational energy landscape of ternaries.
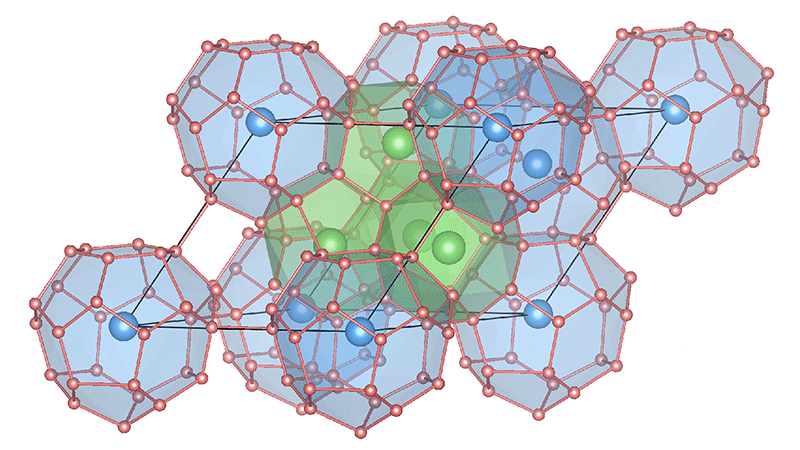
Ma’s group got around some of these difficulties by focusing on the ternary hydride (Fig. 1). This compound can be thought of as the binary hydride, , doped with Li, an electron donor. On its own, would tend to break up into molecules under high pressure, preventing superconductivity. But, based on density-functional-theory (DFT) calculations, the authors claim that the molecules tend to break up with the addition of the extra electrons from Li doping. Preventing helps to stabilize a hydrogen host structure favoring superconductivity like that in . Other groups have considered the benefits of donating electrons to hydrides, and if this approach proves successful, it could contribute to the discovery of high- superconductors using ternary and, potentially, quaternary hydrides.
Going forward, theorists need to figure out how to retain high-temperature superconductivity at lower pressures and, better yet, ambient pressure. To meet this challenge, the predictive power of computational tools will need to be made more robust and accurate, particularly when applied to physical properties under extreme conditions. The current gold standard in reliability is ab initio DFT calculations, which have been a guide for many promising directions to synthesize new superconductors [5].
But standard DFT calculations can be limited in their predictive power. In the case of , for example, conventional DFT calculations predict a much higher pressure (250 GPa) for the stable superconducting structure than what is observed experimentally (150 GPa.) The discrepancy may exist because DFT makes the Born-Oppenheimer approximation—describing a material’s electrons independently of its lattice ions—and it treats the ions as motionless. A recent study, however, suggests the calculations may need to account for the sizable zero-point motion of the hydrogen atoms in . Such quantum effects can stabilize a “quantum crystal” phase that favors superconductivity [8].
Hydride research is producing many exciting results. Of particular note is the recent observation that the hydride superconducts with a of 224 K at 166 GPa [9]—a much lower pressure than expected from theory [6]. As more and more tantalizing candidates are identified, researchers should demand calculations that go beyond the standard approximations in order to have reliable predictions. After all, a new superconductor forecast is only valuable if the material can be synthesized in the lab.
This research is published in Physical Review Letters.
References
- Y. Sun, J. Lv, Y. Xie, H. Lui, and Y. Ma, “Route to a superconducting phase above room temperature in electron-doped hydride compounds under high pressure,” Phys. Rev. Lett. 123, 097001 (2019).
- J. J. Gilman, “Lithium dihydrogen fluoride—An approach to metallic hydrogen,” Phys. Rev. Lett. 26, 546 (1971); N. W. Ashcroft, “Hydrogen dominant metallic alloys: High temperature superconductors?,” 92, 187002 (2004).
- Y. Li, J. Hao, H. Liu, Y. Li, and Y. Ma, “The metallization and superconductivity of dense hydrogen sulfide,” J. Chem. Phys. 140, 174712 (2014).
- A. P. Drozdov, M. I. Eremets, I. A. Troyan, V. Ksenofontov, and S. I. Shylin, “Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system,” Nature 525, 73 (2015).
- J. A. Flores-Livas, L. Boeri, A. Sanna, G. Profeta, R. Arita, and M. Eremets, “A perspective on conventional high-temperature superconductors at high pressure: methods and materials,” arXiv:1905.06693.
- F. Peng, Y. Sun, C. J. Pickard, R. J. Needs, Q. Wu, and Y. Ma, “Hydrogen clathrate structures in rare earth hydrides at high pressures: Possible route to room-temperature superconductivity,” Phys. Rev. Lett. 119, 107001 (2017); H. Liu, I. I. Naumov, R. Hoffmann, N. W. Ashcroft, and R. J. Hemley, “Potential high- superconducting lanthanum and yttrium hydrides at high pressure,” Proc. Natl. Acad. Sci. U.S.A. 114, 6990 (2017).
- M. Somayazulu, M. Ahart, A. K. Mishra, Z. M. Geballe, M. Baldini, Y. Meng, V. V. Struzhkin, and R. J. Hemley, “Evidence for superconductivity above 260 K in lanthanum superhydride at megabar pressures,” Phys. Rev. Lett. 122, 027001 (2019); A. P. Drozdov et al., “Superconductivity at 250 K in lanthanum hydride under high pressures,” Nature 569, 528 (2019).
- I. Errea et al., “Quantum crystal structure in the 250 K superconducting lanthanum hydride,” arXiv:1907.11916.
- I. A. Troyan et al., “Synthesis and superconductivity of yttrium hexahydride ,” arXiv:1908.01534.