Imaging Water Molecules on Metal
Nanoscale interactions between water molecules and a metal surface determine the metal’s affinity to liquids, its electrochemical properties, and its utility as a catalyst. However, testing model predictions is difficult. Scanning tunneling microscopy (STM) can visualize the water layer without disrupting the fragile bonds connecting the molecules, but it can’t always resolve single molecules. Noncontact atomic force microscopy (AFM), meanwhile, has been used mainly to visualize organic molecules, making its ability to nondestructively image water bonding networks uncertain. By combining both techniques, Akitoshi Shiotari, at the University of Tokyo, and colleagues acquired single-molecule-resolution images of water networks on a nickel surface. The researchers expect their work to prompt many more ultrahigh-resolution imaging studies of water adsorption on metals.
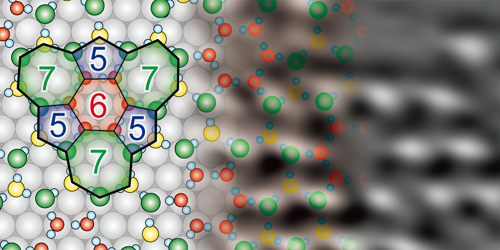
The team exposed a single crystal of nickel to water vapor at 78 K, remobilized the water molecules by warming the sample to 150 K, then cooled it again for imaging. STM images showed that, during annealing, the water molecules arranged themselves into monolayer islands whose periodicity matched the underlying structure of the nickel. AFM images revealed that these islands comprised a network of five-, six-, and seven-sided rings formed from hydrogen bonds between the water molecules. Subsequent force measurements confirmed that the intermolecular structure was not damaged by the imaging process.
The researchers also studied water adsorption at a single-atom step on the nickel surface. They found that the water molecules formed a combination of pentagonal and octagonal rings to accommodate the topography of this defect. Step edges like this are important sites for catalysis, so understanding how they interact with water molecules could eventually improve the yield of, for example, catalytic methods of hydrogen production.
This research is published in Physical Review Materials.
–Marric Stephens
Marric Stephens is a freelance science writer based in Bristol, UK.