Harvesting Energy from Falling Droplets
Two centuries ago, archaeologists digging through the ruins of ancient Babylon made a fascinating discovery—clay tablets written in cuneiform dating back to circa 1800 BCE. These tablets included descriptions of superstitions whereby priests divined the future through the patterns formed by oil poured on water. This ancient text represents the earliest known literature reference to the hydrophobic effect [1]. Now, two independent teams of modern scientists have used this idea, along with other concepts described roughly two millennia ago, to develop remarkably efficient electrical nanogenerators with no moving parts [2, 3]. The nanogenerators are based on several phenomena relating to water and electricity described by the classical Greek philosopher Plato. And the key to making the nanogenerator so efficient is to tag team these phenomena with the hydrophobic effect described in the Babylonian cuneiform tablets.
About 1500 years after the Babylonian reference to the hydrophobic effect, in ancient Athens, Plato speculated in his dialogue Timeaus on the nature of many physical phenomena, including “the flowing of water, the fall of the thunderbolt, and the marvels that are observed about the attraction of amber” [4, 5]. The “attraction of amber” is what we now call triboelectric charging—the electrostatic charging that occurs when two surfaces rub against each other. In fact, our word “electricity” comes from the Greek word for amber—elektron. And unbeknownst to Plato, the “fall of the thunderbolt” is a discharge of energy built up from the triboelectric charging of ice crystals in clouds. The phenomena of triboelectric charging and electrical discharge, along with the flowing behavior of water, are the key ideas behind the new nanogenerators.
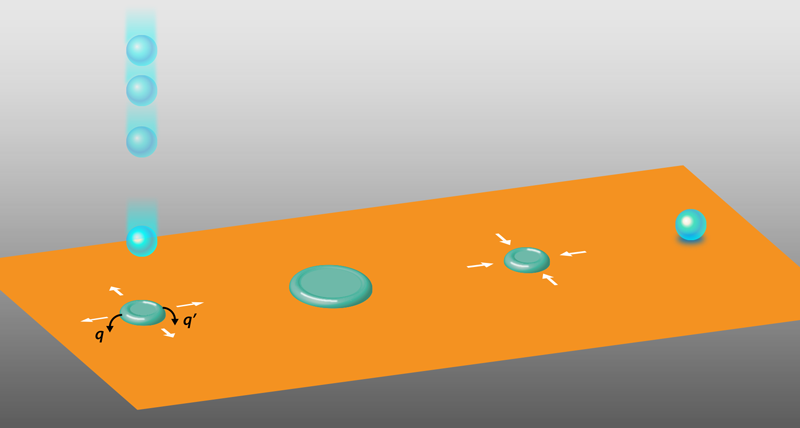
The new nanogenerators work as follows. Water droplets fall onto a dielectric thin film of polytetrafluoroethylene (PTFE) on a conducting plate. Triboelectric charging occurs as the droplet hits the surface, making the droplet positive and the PTFE surface negative. The negative charge on the PTFE induces a positive charge on the conductor below it, creating a capacitor across the dielectric PTFE. Although the charge transfer with each drop is small, the total charge on the PTFE surface builds up after contacting many droplets. The charge eventually saturates, causing the capacitor to carry a significant amount of energy that can be used to power a device. Beyond charging the PTFE surface, the water droplets play another role. As each droplet spreads on the PTFE surface, it temporarily touches an electrode located above the surface, forming a conducting bridge between the PTFE surface and the electrode. This electrode is also electrically connected to the charged conductor that serves as the bottom plate of the capacitor. Each droplet thereby serves as a switch that discharges the capacitor to create a current that powers a load. But if this process were the complete story, the device would not be effective [2, 3].
Herein lies the problem with traditional triboelectric nanogenerators: if a capacitor is discharged to use the stored energy, many further droplet contacts would be needed to recharge it. Previous nanogenerators did not involve a capacitor but, rather, converted the energy from each individual droplet to electrical current; this setup works but produces a very small amount of current [6].
Now, Wanghuai Xu of the City University of Hong Kong and of the University of Science and Technology of China and colleagues have employed a clever use of the hydrophobic effect to automatically recharge the capacitor after each discharge [2]. On a hydrophobic surface such as PTFE, water tends to “bead up,” leading to little contact area with the surface. Yet when a falling droplet first hits the surface, its vertical kinetic energy is transformed to lateral kinetic energy that causes it to spread out. As discussed above, the spreading causes the droplet to touch the electrode and discharge the capacitor. Furthermore, this spreading increases the droplet’s potential energy because of the hydrophobic interaction between the droplet and the surface—much like pulling on a spring increases its potential energy. After the kinetic energy is lost, this potential energy causes the spread droplet to recede and bead up (i.e., assume its equilibrium configuration). While the droplet is receding, the capacitor becomes recharged. Hao Wu of the University of Twente in the Netherlands and of South China Normal University and colleagues [3] developed a quantitative model describing how energy from the retracting spring recharges the capacitor: As the droplet retracts, its decreasing size increases its surface charge density, driving current in reverse to recharge the capacitor.
These recent advancements are exciting for their application as nanogenerators, which harvest enough electricity from ambient energy sources (e.g., rain droplets) to power small electronic devices. Thus, the nanogenerators can be effective for applications such as powering sensors in remote locations [6]. They may also help provide a route to understanding the physical mechanisms behind triboelectric charging.
Surprisingly, after more than 2000 years of inquiry, we don’t know much more about triboelectric charging [7] than Plato’s vague notions in Timaeus that lightning and the electrostatic charging of amber are related to “the fact that objects push one another round” and “are divided or combined” [4]. What are these “objects” being pushed around as two surfaces contact and become electrostatically charged? We don’t know the answer—they could be electrons or some type of ions. We don’t know what material properties act to “push” electrons or ions from one surface to another. And we don’t understand what role rubbing plays in triboelectric charging. Does it simply increase the surface area of contact, or does it provide energy to “divide” the charge?
When we think of triboelectric charging, we usually think of charging between two solid surfaces. However, liquid-solid triboelectric charging, as in these nanogenerators, is likely simpler and more controllable than its solid-solid counterpart. Interactions between solid surfaces are complicated; roughness reduces the contact area between surfaces and concentrates force at certain points, breaking bonds [8–10]. Liquids, on the other hand, deform to make full contact with a solid surface, and their flow avoids the concentration of force that breaks bonds. Perhaps liquid-solid triboelectric charging will be the key to more highly controlled experiments that finally unlock the mysteries of triboelectric charging.
This research is published in Physical Review Letters and Nature.
References
- D. Tabor, “Babylonian lecanomancy: An ancient text on the spreading of oil on water,” J. Colloid Interface Sci. 75, 240 (1980).
- W. Xu et al., “A droplet-based electricity generator with high instantaneous power density,” Nature 578, 392 (2020).
- H. Wu et al., “Energy harvesting from drops impacting onto charged surfaces,” Phys. Rev. Lett. 125, 078301 (2020).
- Plato, Timaeus. Translated by B. Jowett (Project Gutenberg Ebook, 2008).
- P. Iversen and D. J. Lacks, “A life of its own: The tenuous connection between Thales of Miletus and the study of electrostatic charging,” J. Electrostat. 70, 309 (2012).
- Z.-H. Lin et al., “Harvesting water drop energy by a sequential contact-electrification and electrostatic-induction process,” Adv. Mater. 26, 4690 (2014).
- D. J. Lacks and T. Shinbrot, “Long-standing and unresolved issues in triboelectric charging,” Nat. Rev. Chem. 3, 465 (2019).
- S. Rajupet et al., “Particle adhesion to rough surfaces,” Phys. Rev. E 102, 012904 (2020).
- M. K. Beyer and H. Clausen-Schaumann, “Mechanochemistry: The mechanical activation of covalent bonds,” Chem. Rev. 105, 2921 (2005).
- B. Baytekin et al., “Mechanically driven activation of polyaniline into its conductive form,” Angew. Chem. 126, 7066 (2014).