In Hot Pursuit of 21st Century Cooling
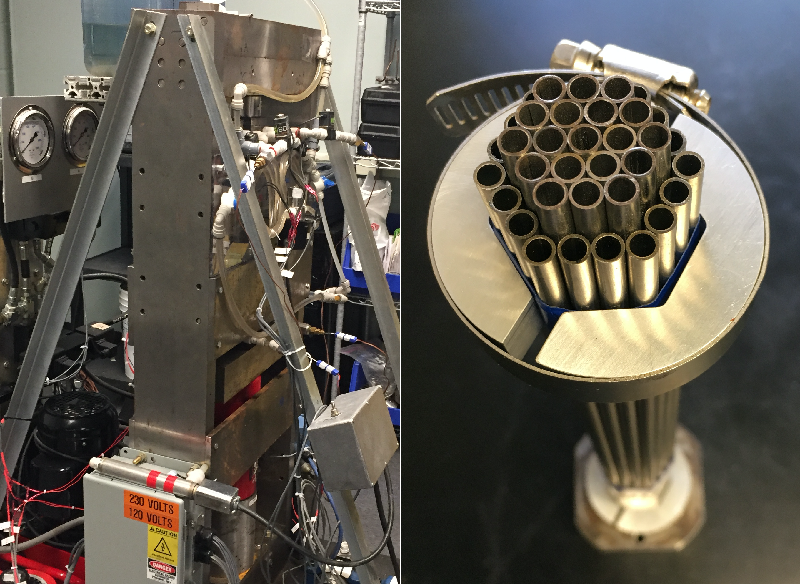
It’s not exactly a looker, but a five-foot tall, A-shaped engineering rig at the University of Maryland’s College Park campus could potentially evolve into a partial solution for a warming planet. At the heart of this refrigeration prototype is a material that gets cold just by being squeezed and released. It’s one of many so-called caloric materials that could provide for the world’s rapidly expanding cooling needs without exacerbating climate change. These materials cool in response to pressure, electric fields, or magnetic fields. Completely absent from this solid-state cooling concept are the volatile liquid refrigerants that are the lifeblood for just about all current cooling technologies and that are important contributors to global warming.
As the world’s billions of air conditioners, refrigerators, chillers, and dehumidifiers age and become throwaways, their refrigerants mostly leak into the atmosphere, where they trap solar radiation far more powerfully than carbon dioxide. And every day, the increasingly sweaty citizens of the planet install about 260,000 new air-conditioning (AC) units. At that rate, the number of ACs will likely balloon from the current 1.2 billion or so to 4.5 billion by 2050 [1]. The vapor-compression systems inside cooling appliances already consume about 20% of global electricity, the generation of which is a top source of humanity’s carbon dioxide emissions. Without a planet-wide redo of cooling technology, the explosive growth expected in cooling demand and supply is destined to make global warming worse. And that, in turn, will increase demand for more cooling.
It’s a vicious cycle, already shredding other facets of the world’s response to climate change. “All new electricity capacity created from additional solar energy [in 2018] was completely eclipsed by new AC units being plugged in,” says Jessica Brown of ClimateWorks Foundation, a San Francisco philanthropy that funds efforts to fight climate change.
To interrupt this cycle, University of Maryland materials scientist Ichiro Takeuchi—co-creator of the A-shaped refrigeration testing setup—and other researchers, technologists, and entrepreneurs around the world are developing caloric materials. They hope to usher cooling technology through a shift akin to the transition from vacuum tubes to transistors or the ongoing replacement of incandescent bulbs by LEDs. To get there, they must find materials with a hard-to-assemble suite of properties: sufficient cooling power, superior energy efficiency, enough failure resistance, adequate availability of ingredients, and low enough cost. That’s a lot to ask for in one material, but as the need for a solution has intensified, researchers continue searching for the caloric material that has it all
Cooling Without a Gas
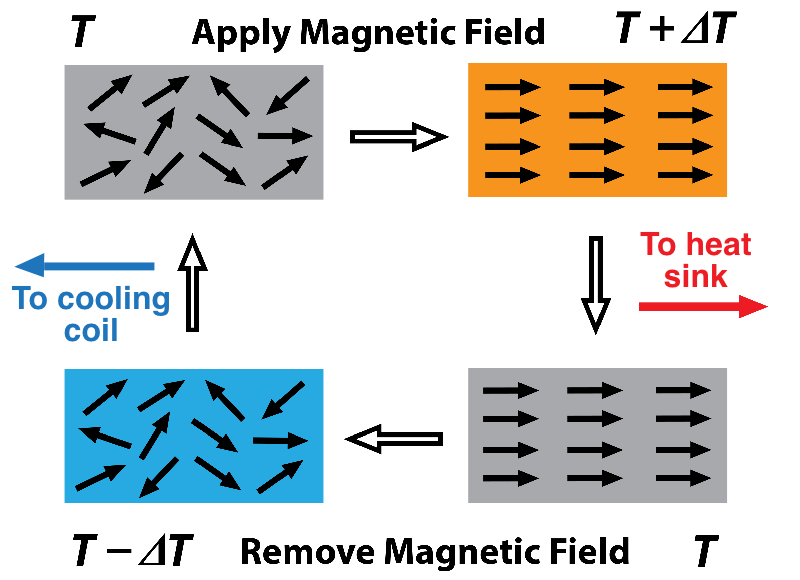
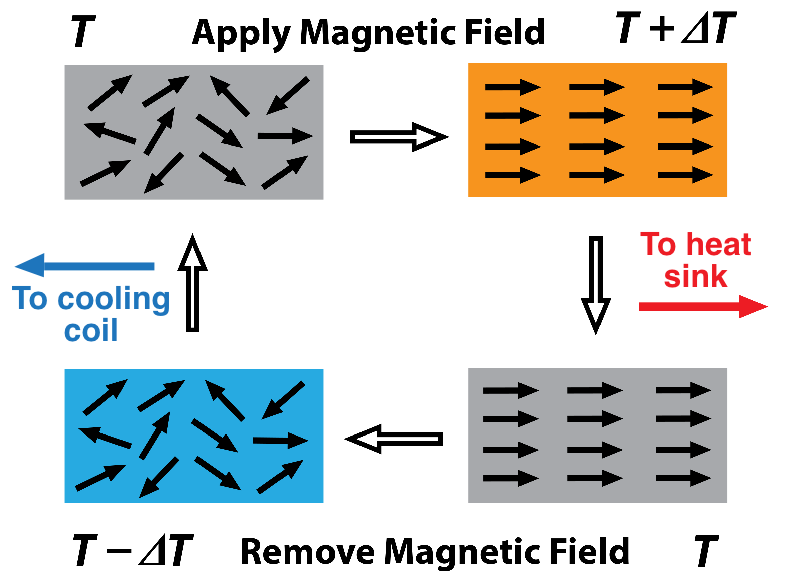
The growing roster of candidate caloric materials includes metals, ceramics, and plastics. The temperature variations in caloric materials result from changes in the entropy of the microstructures of the materials as they are subjected to a cycling magnetic, electric, or stress field. For example, you can reduce the magnetic entropy in a magnetocaloric material by aligning the magnetic domains with an applied magnetic field. The entropy of the atomic lattice vibrations increases to compensate, which causes heating. Remove the order-enforcing field so that the domains become more randomly arranged (higher entropy), and the temperature drops because the domains soak up entropy from the vibrating lattice.
The trick is to shunt the heat generated during the low-magnetic-entropy phase of the cycle to the outside using a heat-transfer fluid. Then, during the high-magnetic-entropy phase, the heat-exchange system has to change direction to route the now chilled heat-transfer fluid into cooling coils of a refrigeration chamber or AC unit.
“The basic science is there. We know how to do it,” says Vitalij Pecharsky of the U.S. Department of Energy’s Ames Laboratory in Iowa. “We just don’t know how to do it inexpensively.” For example, in the subfield of magnetocaloric materials, no one has come up with a sufficiently fatigue-resistant material that can produce enough cooling when driven by a small enough magnetic field—all at an affordable price point, says Pecharsky. But he and other caloric materials experts are cautiously optimistic that progress will come with time and sufficient commitment to solving the engineering challenges.
The Many Flavors of Caloric Materials
Researchers differ on which type of caloric material—electro, magneto, or elasto—holds the most promise. Takeuchi is now betting on a class of copper-based materials that are part of the so-called shape-memory alloy (SMA) family, which can undergo elastocaloric temperature changes with relatively gentle squeezing. Takeuchi says he obtained samples of the alloys from a Japanese company that is developing them to help squelch earthquake motions in buildings. The materials should become available in large quantities and at affordable prices, he says. “You can imagine if the materials are mass produced, then we are in business.”
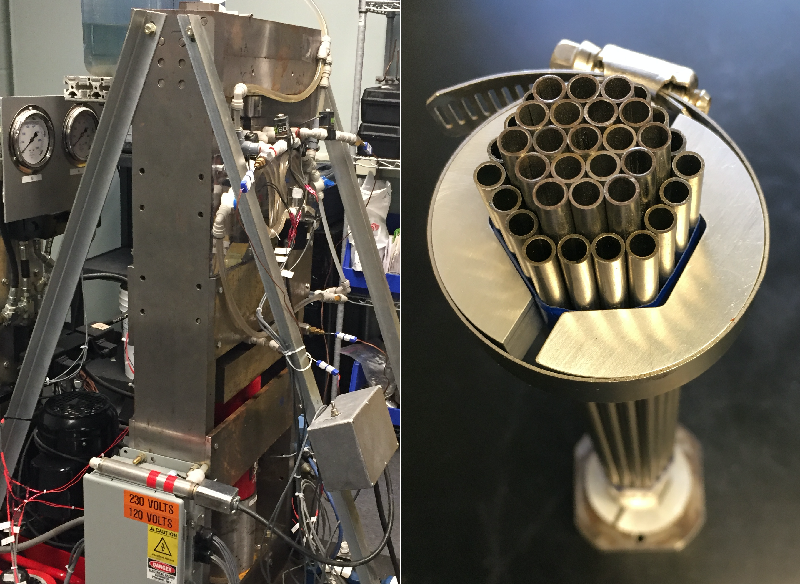
For now, he and graduate student David Catalini are experimenting with clusters of hollow, pencil-sized tubes of SMAs in their A-shaped rig in an effort to develop prototype appliances with the modest cooling demands of, say, a wine chiller. The hollow format of these tube clusters makes it easy to flow a heat-exchanging fluid through them as the tubes undergo cycles of being squeezed (by a piston) and released.
Materials scientist Qiming Zhang of Pennsylvania State University (Penn State), University Park, is an elder of the electrocalorics subfield. Electric fields don’t penetrate far into most solids, so electrocaloric materials are generally used in thin structures that are best suited to small-scale devices, such as those made for cooling electronic components. For the past 15 years, Zhang has been trying to develop electrocaloric ceramics and polymers into cooling materials that are capable enough, cheap enough, and durable enough to be practical. In December, at the Materials Research Society (MRS) meeting in Boston, he reported progress in generating large electrocaloric effects (up to 20 °C) with a polymer using weak electric fields.
Pecharsky is confident about magnetocaloric materials. In the 1990s, he and colleagues teamed up with the Milwaukee-based Astronautics Corporation. The collaboration produced a prototype magnetocaloric refrigerator based on gadolinium alloys rotating in and out of a magnetic field. The achievement provided a proof-of-principle that caloric refrigeration was possible, and Astronautics tried to develop a commercial system. But the company was never able to sufficiently reduce the cost of producing the required magnetic field and make the system competitive with vapor-compression appliances. So they finally abandoned the project a few years ago, says Astronautics engineer Steve Russek. Pecharsky accepts that the road to magnetocaloric refrigeration will be a long one, but he remains convinced that the current show-stoppers can be overcome.
Success under Pressure
Barocal, a startup company in Cambridge, UK, is hoping to accelerate technology development with its proprietary barocaloric effect (BCE) materials. These elastocaloric materials warm and cool in response to hydrostatic pressure changes. Barocaloric materials recently got a status boost when, in November, Barocal was named among the eight finalists from 139 competitors in the Global Cooling Prize challenge. Largely funded by billionaire Richard Branson, the Prize aims to spur development of “super-efficient and climate-friendly residential cooling solutions.” Barocal received $200,000 and the chance to win the one million dollar top prize the organizers expect to award later this year.
At the heart of Barocal’s prototype design are so-called “plastic crystals,” such as neopentyl glycol (NPG), which exhibit what the company calls “colossal barocaloric cooling effects.” William Averdieck, the company’s managing director and a mechanical engineer at Cambridge University, claims that Barocal’s best material undergoes a whopping temperature change ( ΔT) of up to 50 °C when pressed with 1000 atmospheres of pressure.
In April, the company’s director of research, Xaviar Moya, and colleagues reported that the large barocaloric effects they measured in NPG derive from the ability of the globular molecules to undergo dramatic and reversible volume-reducing rearrangements under pressure [2]. The researchers noted that the pressure-induced entropy and thermal changes they observed were 10 times better than those measured in state-of-the-art barocaloric materials and were “comparable to those observed in the standard commercial hydrofluorocarbon refrigerant R134a.” At about the same time, another team reported similar results for NPG [3].
Averdieck admits that a 1000-atmosphere operating pressure is impractically high. Even so, he says, the extremely high ΔT for NPG “gives us confidence to proceed.” The company’s current design features an oil-based suspension of plastic crystal particles in a closed chamber whose pressure is cycled up and down by a piston.
More to Come
Researchers continue to pursue new types of caloric materials. For example, some are investigating “multiferroic” materials, which can cool in response to several applied fields, such as a magnetic field and pressure, not just one. And at the MRS meeting, researchers from Penn State and Case Western Reserve University in Cleveland described their use of machine-learning techniques to computationally screen 1034 potential iron alloys, composed variously of 18 elements, for magnetocaloric properties.
Takeuchi is still placing his bets on elastocaloric alloys. He was optimistic enough even a decade ago that he started a company, Maryland Energy and Sensor Technology, with the goal of commercializing elastocaloric technologies. “We are planning on demonstrating an elastocaloric wine cooler” this year, he says. In a sign of Takeuchi’s optimism, several wine-cooler housings waiting to be fitted with elastocaloric cooling modules sit against a wall in his lab. The incentives to continue innovating are hard to ignore; in the six minutes it might have taken you to read this article, more than a thousand brand-new air-conditioners started humming.
–Ivan Amato
Ivan Amato, a science writer in Hyattsville, Maryland, is editor of The Moonshot Catalog and a documentarian for the U.S. Defense Advanced Research Projects Agency.
References
- I. Campbell et al., Solving the Global Cooling Challenge, report from the Rocky Mountain Institute (2018).
- P. Lloveras et al., “Colossal barocaloric effects near room temperature in plastic crystals of neopentylglycol,” Nat. Commun. 10, 1803 (2019).
- B. Li et al., “Colossal barocaloric effects in plastic crystals,” Nature 567, 506 (2019).