Bouncing Droplets Reveal New Leidenfrost Effect
Sprinkle some water on a sufficiently hot pan, and the droplets skitter across the surface, insulated from rapid boiling by a layer of water vapor upon which they float. New experiments show that this “Leidenfrost” effect can occur between two droplets of differing liquids [1]. A gas layer develops between the droplets, causing them to bounce off each other for seconds or minutes before finally merging. The researchers behind the experiments say that their work could help improve understanding of the interactions of fuel droplets with hot engine surfaces.
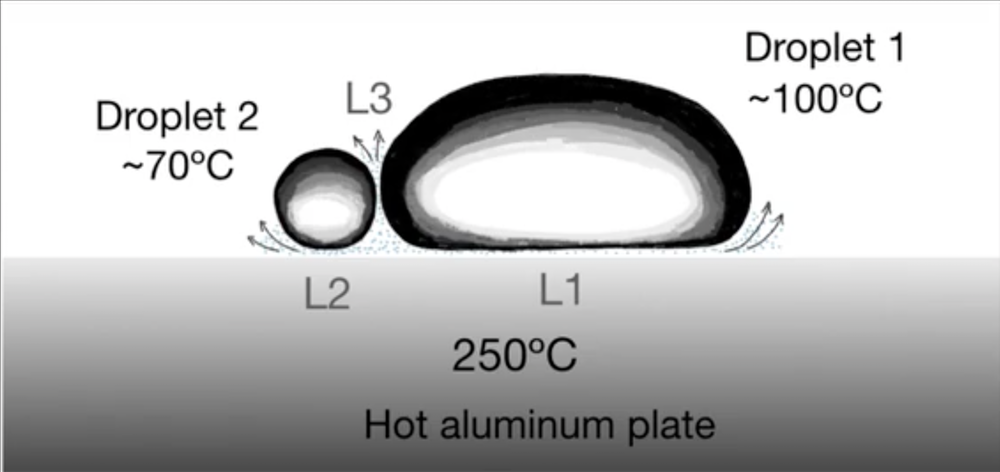
In the experiments, performed by Felipe Pacheco-Vázquez of the Autonomous University of Puebla, Mexico, and his colleagues, the researchers used a metal plate with a slightly concave top surface and heated it to 250∘C. They placed a series of water droplets onto the surface and observed them merging into a blob at the lowest point in the center. However, when the team added an ethanol droplet, it did not immediately merge but instead bounced many times against the larger water droplet. Based on results from ten different liquids, the team suggests that the rebounds are caused by a vapor layer between droplets, similar to the one that each droplet produces underneath itself. According to this theory, there are three separate Leidenfrost layers involved in the two droplets, so the researchers call it a “triple Leidenfrost” effect.
In these conditions, the liquids are essentially at their boiling points, so for example, water would be at about 93∘C (given the altitude of the lab in Puebla, Mexico) and ethanol at about 72∘C. This large temperature difference can generate a Leidenfrost layer between droplets. Eventually, however, the more rapidly evaporating ethanol droplet becomes so small that it can’t generate enough vapor to withstand the attraction to the water. In other cases, when the difference in boiling points is too small for a Leidenfrost effect between droplets, they merge without any bouncing.
–David Ehrenstein
David Ehrenstein is a Senior Editor for Physics Magazine.
References
- F. Pacheco-Vázquez et al., “Triple Leidenfrost effect: Preventing coalescence of drops on a hot plate,” Phys. Rev. Lett. 127, 204501 (2021).