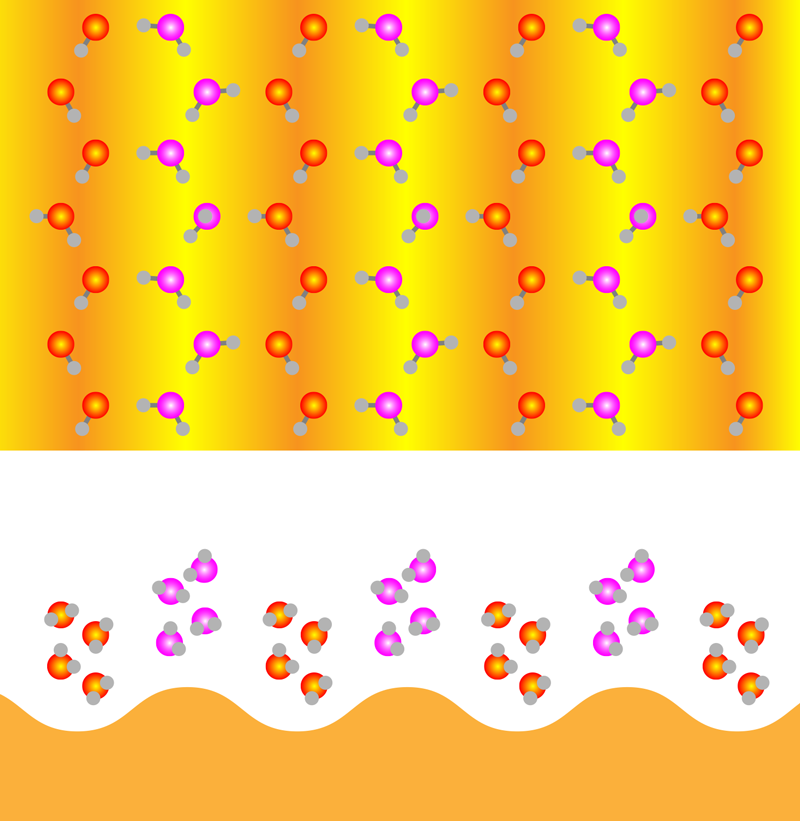
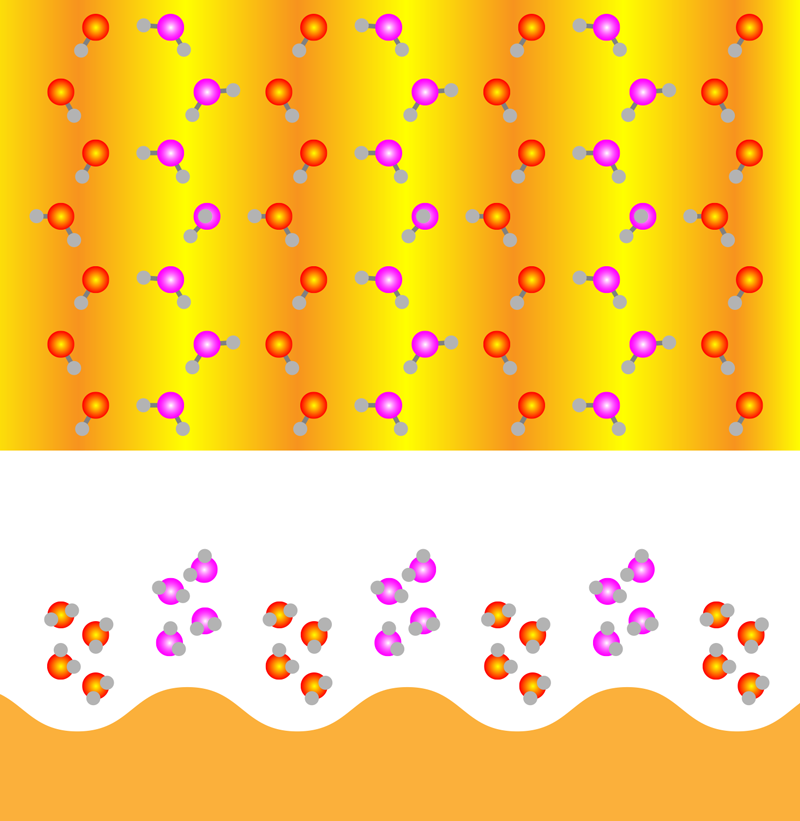
Hydrophobic Ice More Common than Thought
Water is a liquid, but it can form a wide variety of ordered molecular arrangements when it comes in contact with another material. The structure that water layers adopt at the interface is important for a wide variety of phenomena including corrosion, protein folding, ice nucleation, and antifog coatings. For example, the interactions of water with the hydrophilic and hydrophobic portions of a protein influence how the protein folds and thus how it functions [1]. Because water’s structure near an interface is known to be the result of a delicate balance of several competing factors, it might seem hopeless to predict which structure will develop in a particular situation [2]. However, new results by Ying Jiang of Peking University and colleagues suggest that a form of 2D ice—one that strangely behaves like a “water-fearing,” or hydrophobic, surface—might arise in more situations than previously thought [3]. The observations should help theorists in modeling the shape of water in all its forms.
Two key features that influence the structure of water are its tendency to form four bonds and the highly directional nature of those bonds. In the bulk, both features are accommodated by the well-known tetrahedral bonding geometry that describes hexagonal ice and that approximates the behavior of liquid water. However, at the interfaces of water with other materials, it is typically not possible to satisfy both structure constraints, and the nature of this “frustration” depends on whether the water-water interaction or the water-substrate interaction is stronger. These cases correspond to interactions with hydrophobic or hydrophilic surfaces, respectively.
An example of how these factors come into play was highlighted by simulations in 1997 that looked at water confined between two planar, hydrophobic surfaces. Those simulations predicted a new crystalline ice polymorph, called bilayer hexagonal ice (BHI), that was unlike any previously identified type of ice [4]. Constrained to be only two molecular layers thick and without the ability to form strong bonds with the substrate, the water molecules in BHI are forced to give up the tetrahedral-bonding geometry found in most ices. Instead, the molecules assemble in two flat layers where every molecule forms four hydrogen bonds—three hydrogen bonds with their neighbors in the same layer and one hydrogen bond with a molecule directly opposite it in the other layer. This configuration is predicted to have the lowest free energy—even though the hydrogen bonds are distorted. The energy is minimized because BHI maximizes the total number of hydrogen bonds for the system.
Eventually, this bilayer ice was also found experimentally—not in a confined geometry but in water films growing on flat, hydrophobic surfaces in a vacuum [5, 6]. Surprisingly, these water films were hydrophobic, as nearly all the water molecules in the BHI structure have a complete set of four bonds, so there are no binding sites for new molecules to attach on top of the film. Instead, new molecules can bond at the edges, causing the film to extend over the surface. This observed wetting behavior was unexpected, as it usually occurs on hydrophilic surfaces.
Jiang and colleagues have now found the BHI structure in other places—where the surfaces are neither strongly hydrophobic nor perfectly flat [3]. In their study, they used scanning tunneling microscopy and atomic force microscopy to image thin water films on two types of gold surfaces—Au(110) and Au(100)—at 5 K. The team achieved stunning detail by placing a CO molecule on the tip of the scanning probe. This “molecularly sharp” tip resolved the hydrogen-bonding arrangement for water films that were one and two layers thick.
For Au(110), the water-substrate interaction is a little less strong than the water-water interaction, thus making this gold surface slightly hydrophobic. In addition, Au(110) forms a corrugated pattern when in an ultrahigh vacuum that leaves it looking like a farmer’s field that has recently been plowed. The team observed that the first water monolayer adsorbed on this surface with rows of water molecules aligning with the surface’s furrows and ridges. The molecules in the furrows had one hydrogen atom pointing toward the surface, forming a weak bond—an “H-down” configuration observed on many hydrophilic surfaces. The molecules on top of the ridges adsorbed largely parallel to the surface. Viewed from above, this monolayer film has a hexagonal arrangement reminiscent of a single layer in bulk crystalline ice, despite the corrugation of the substrate. However, unlike a layer from crystalline ice, this monolayer presented few binding sites for any water molecules attempting to adsorb on top of it.
When more water was added to the surface, the first monolayer was forced to adapt. For two-layer films, the team observed a significant rearrangement of the hydrogen-bonding network in the film and the emergence of a close analog of the BHI structure found on flat, hydrophobic surfaces (Fig. 1). While one out of every four molecules in the furrows retained the H-down configuration, the rest flipped into an “H-up” configuration to bind to water molecules in the layer above. For molecules in the second layer, most molecules also had four hydrogen bonds, with only a few having binding sites available for any additional water layers. Unlike previous results, this BHI was corrugated to conform to the substrate. To investigate the commonness of BHI formation, the team also imaged water layers on an Au(100) surface, which is flatter and more hydrophobic than Au(110). Again, the BHI was observed with only slight differences that could be traced to the influence of the substrate.
The formation of BHI even in nonideal circumstances suggests that this structural motif for water at interfaces will play a bigger role than previously anticipated. The new results will also help researchers who are investigating water by providing them with benchmarks against which future simulation results can be checked. However, much work remains to be done. A key deficit in our understanding of the structure of water at interfaces remains how to connect the exquisitely detailed measurements of model systems with real-world applications that involve dirty surfaces with multilayers of water at higher temperatures. Progress on this front has been slow, but new experimental techniques and advances in theory, modeling, and simulation are all encouraging [7, 8].
References
- N. Giovambattista et al., “Hydrophobicity of protein surfaces: Separating geometry from chemistry,” Proc. Natl. Acad. Sci. U.S.A. 105, 2274 (2008).
- A. Hodgson and S. Haq, “Water adsorption and the wetting of metal surfaces,” Surf. Sci. Rep. 64, 381 (2009).
- P. Yang et al., “Robustness of bilayer hexagonal ice against surface symmetry and corrugation,” Phys. Rev. Lett. 129, 046001 (2022).
- K. Koga et al., “Freezing of confined water: A bilayer ice phase in hydrophobic nanopores,” Phys. Rev. Lett. 79, 5262 (1997).
- G. A. Kimmel et al., “No confinement needed: Observation of a metastable hydrophobic wetting two-layer ice on graphene,” J. Am. Chem. Soc. 131, 12838 (2009).
- D. Stacchiola et al., “Water nucleation on gold: Existence of a unique double bilayer,” J. Phys. Chem. C 113, 15102 (2009).
- J. J. Velasco-Velez et al., “The structure of interfacial water on gold electrodes studied by x-ray absorption spectroscopy,” Science 346, 831 (2014).
- J. Chen et al., “Two dimensional ice from first principles: Structures and phase transitions,” Phys. Rev. Lett. 116, 025501 (2016).