How Cells Move through Narrow Spaces
The movement of cells is essential for embryo development and wound healing. A study of individual human cells moving on a micropatterned surface reveals some of the basic principles governing this movement and shows how cells adapt their shape and behavior to the geometry of their surroundings [1]. The researchers developed a theoretical model, based on their experimental findings, that could be used to study and predict cell movement in more complex environments.
The shapes of animal cells are controlled in part by a web of protein filaments called the cytoskeleton, which can be rearranged by the cell to drive motion. For example, a cell can begin moving by creating a protrusion that bulges out from its surface. Such movement depends on the cell’s adhesion to the surrounding surfaces and on the formation of an asymmetrical arrangement of the cytoskeleton, referred to by biologists as polarity, which drives the growth of protrusions. The motion is also affected by the internal structures of the cell, especially the nucleus, which is less compressible than the fluid cytoplasm.
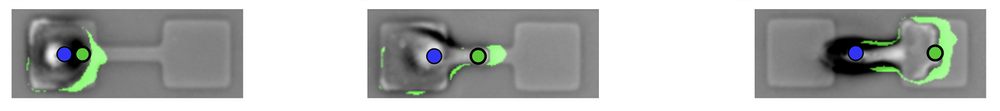
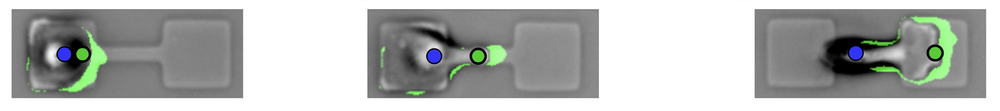
To understand these various influences, researchers have studied how cells move on micropatterned surfaces with simple, geometric confining structures such as islands, channels, and grooves [2]. David Brückner and his co-workers at the Ludwig-Maximilian University of Munich (LMU) previously looked at a human cancer cell migrating between two “sticky” islands connected by a narrow bridge [3]. They found that the cell develops a thin protrusion that reaches across the constriction and then draws the rest of the cell across.
Now the Munich team has extended that work by taking account of the shape changes of the entire cell. In the previous work, as well as in that of other researchers, only the motion of the nucleus was tracked. Gathering this more comprehensive picture of cell migration, says LMU team member Chase Broedersz, relied on a machine-learning algorithm that tracked the shapes of hundreds of cells over several days as each one migrated back and forth across a bridge. Each bridge connected two square patches (37 × 37 micrometers) of a surface coated with the adhesive protein fibronectin. The team used machine learning to reliably identify the cell edges in the microscope images.
“Analyzing the full shape dynamics of cells made it possible for us to disentangle how protrusions and the polarity dynamics inferred from them are coupled to the motion of the nucleus,” says Broedersz. The researchers found that a bridge-crossing protrusion usually allows the main body of the cell to “flow” across to the other patch before the nucleus is subsequently pulled over.
A key question was whether, as cells move, they actively change their shapes to adapt to the geometry of their surroundings, or whether they are passively shaped by them, like water taking the shape of a containing vessel. The researchers concluded that cells respond actively and that the bridge-crossing involves a kind of switch in the internal state of the cell between a fairly aimless “exploratory mode” and a directional “growth mode” dominated by the single protrusion along the bridge. It is as if the cell senses the confining shape and elects to change shape accordingly. “This adaptive response to confinement has not previously been recognized,” says Broedersz.
The team’s model, informed by the experimental data, successfully predicted how the movement would change as the bridge width was altered. The researchers now plan to check their model’s predictions for cell migration in more complex environments, such as in 3D confinement or in mazes—and perhaps ultimately, in living tissues.
“The authors have done a fine job in coming up with their phenomenological approach to the problem,” says biophysicist Herbert Levine of Northeastern University in Boston. “The novelty thus lies in the data-driven theoretical treatment, which is more complete and more quantitative than in their previous work.”
But Levine cautions that the case of cells moving in 3D in real tissues is much more complicated and qualitatively different from movement on a flat surface. Bioengineer David Caballero of the University of Minho in Portugal agrees. The 3D microenvironment in real tissues “triggers cell responses that are completely different from those observed in 2D,” he says.
Broedersz responds that cell migration through confining geometries still occurs in living organisms, for example, as immune cells move through the extracellular matrix of tissues or as cancer cells migrate from tumors during metastasis. If the adaptation to the confining geometry observed in the experiments also occurs in those situations, he says, perhaps the molecular mechanisms that underpin it could provide targets for cancer drugs.
Correction (20 Sept. 2022): The video caption has been updated to provide the length of the scale bar.
–Philip Ball
Philip Ball is a freelance science writer in London. His latest book is How Life Works (Picador, 2024).
References
- D. B. Brückner et al., “Geometry adaptation of protrusion and polarity dynamics in confined cell migration,” Phys. Rev. X 12, 031041 (2022).
- C. D. Paul et al., “Engineered Models of Confined Cell Migration,” Annu. Rev. Biomed. Eng. 18, 159 (2016).
- D. B. Brückner et al., “Stochastic nonlinear dynamics of confined cell migration in two-state systems,” Nat. Phys. 15, 595 (2019).