How Sea Spray Might Disperse Large Particles
The bursting bubbles in ocean foam produce a mist of droplets that can contain enhanced concentrations of ocean pollutant particles. In single-bubble lab experiments, researchers have now shown that the inclusion of a particular particle in an airborne droplet depends on some details of the fluid motion that occurs when the droplet forms [1]. The researchers measured the efficiency with which particles are transferred to droplets and also ran simulations of the fluid motion. Their model predicts that the airborne droplets should contain more large particles, such as microplastics, and fewer smaller particles, such as viruses, than expected based on previous theories.
The droplets produced by bursting air bubbles in ocean foam provide the atmosphere’s main source of ocean-originated particles, including pollutants and bacteria, and environmental scientists want to understand this ocean-to-atmosphere transfer process in detail. Experiments in the 1970s showed that bursting bubbles transport pathogens into the environment and also that the droplets produced contain these particles at high concentrations.
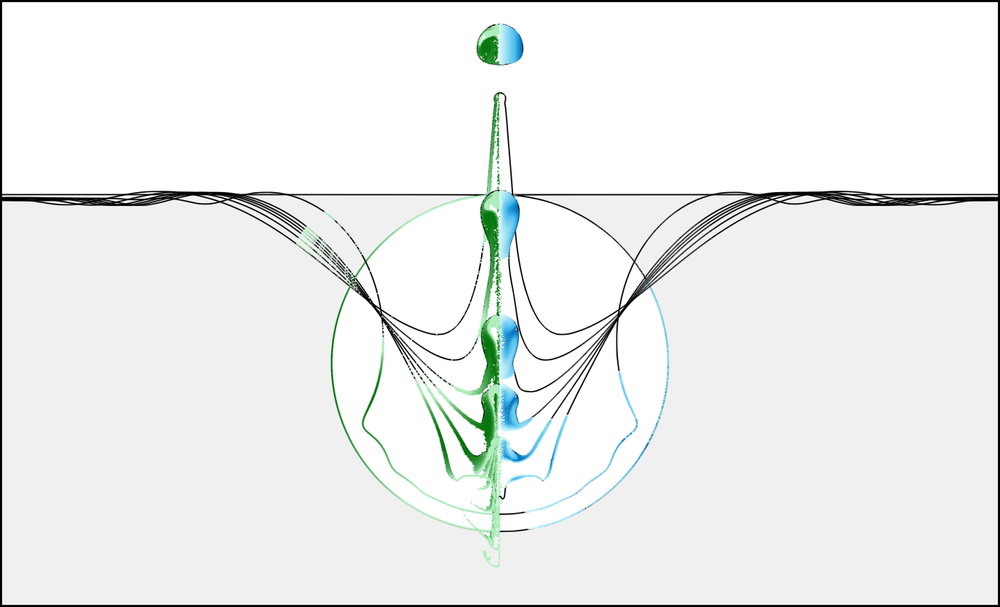
The explanation was that as a bubble moves around within the fluid, it accumulates particles that stick to it. When the bubble bursts at the water’s surface, a water column briefly forms there and then breaks up into small droplets packed with particles that the bubble collected. Researchers have found that the top droplet—the one that is ejected the farthest and that is most likely to remain airborne—forms entirely from fluid originating from a thin layer, called the microlayer, surrounding the original bubble.
According to previous theories, this mechanism for concentrating particles into the top droplet requires particles whose diameters are smaller than the microlayer’s thickness; larger particles wouldn’t be swept with the flow into the droplet. However, atmospheric researchers have found larger particles like microplastics in sea sprays, presumably released from bursting bubbles.
To test whether this mechanism works for larger particles, James Bird of Boston University (BU) and his colleagues filled a container with water mixed with 15- or 30-micrometer-diameter plastic beads—much larger than the typical microlayer thickness of less than half a micrometer. Then, using a syringe, they injected an air bubble at the bottom of the container and watched it rise and ultimately burst and create droplets. A glass slide above the water captured the top ejected droplet and its constituent beads.
The accepted theory predicts that none of these large particles will end up in the droplet, but “when we tested this theory, to our surprise, we found that even particles many times larger than the fluid layer became highly concentrated inside the drops,” Bird says. In addition, the researchers saw wide variations in the number of particles in each droplet.
Previous models assumed that the microlayer is a uniform spherical shell. But the team’s numerical simulations revealed that its thickness varies around the bubble and that it doesn’t cover the bubble’s entire surface area. When the bubble bursts, some regions of the microlayer become stretched and thinner, while others become compressed and thicker. These thickness variations following bursting determine which particles end up in the droplet—at any given location, the thicker the microlayer, the larger the surviving particles can be. The researchers say that the nonuniform thickness also adds some bubble-to-bubble variability that explains the wide variation in the number of particles in each droplet. Each particle needs to be at the right location around the bubble in order to be thrust into the droplet.
The researchers’ work also suggests an effect of bubble size: The microlayers of smaller bubbles cover a smaller fraction of the full sphere than those of larger bubbles, according to the simulations, so they deliver fewer particles to the droplets. These particles are more likely to be small because smaller bubbles have thinner microlayers. So the tiniest particles, like viruses, are less likely to reach droplets than previously expected. “This result is totally unexpected from past theory, which assumes a uniform microlayer all around the bubble,” says BU team member and graduate student Lena Dubitsky.
The study concludes that the position of a particle on the bubble determines whether it finds its way into the droplet. What determines a particle’s initial position on a bubble’s surface, however, remains an open question.
“This new approach has implications for the generation of sea-spray aerosol and in the dispersal of pathogens and pollutants,” says Jose Manuel Gordillo, a fluid mechanics researcher at the University of Seville, Spain. The model may inform new predictions of disease outbreaks or pollution transport, he says.
–Rachel Berkowitz
Rachel Berkowitz is a Corresponding Editor for Physics Magazine based in Vancouver, Canada.
References
- L. Dubitsky et al., “Enrichment of scavenged particles in jet drops determined by bubble size and particle position,” Phys. Rev. Lett. 130, 054001 (2023).