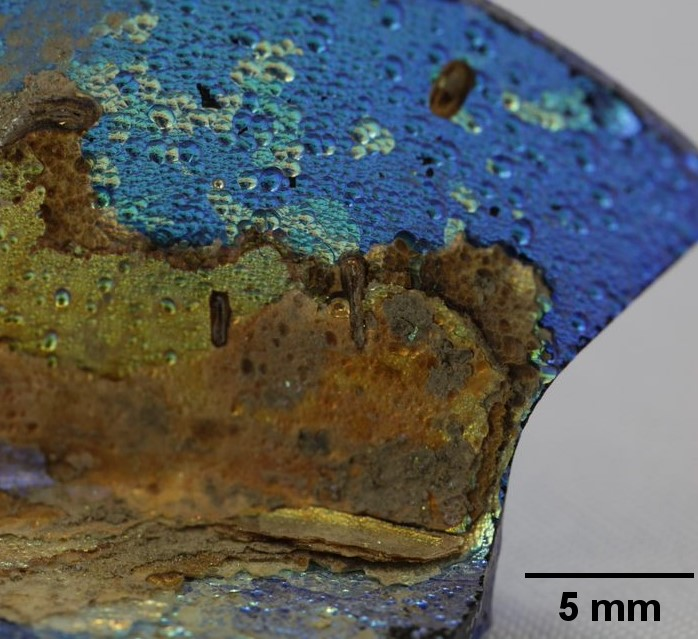
How a Piece of Roman Glass Became a Photonic Crystal
The ancient Roman city of Aquileia was situated close to Italy’s modern border with Slovenia. Over the centuries since its founding in 181 BCE, Aquileia suffered floods, earthquakes, sieges, and sackings. Little remains of this ancient city of 100,000 inhabitants, but archaeologists have uncovered relics from that early period. One such specimen is a glass shard discovered in 2012 on farmland in the outskirts of the modern city of Aquileia. The shard is striking in its coloration: an iridescent surface of deep blue and shiny gold atop a substrate of dark green. Now, after subjecting the shard to a string of chemical and physical tests, Giulia Guidetti of Tufts University, Massachusetts, and her collaborators have identified the origin of the shard’s appearance: a chemical transformation of the amorphous glass into a nanolayered material, a photonic crystal [1].
Glassmaking was invented independently by several Bronze Age civilizations (3300 BCE to 1200 BCE), including those of ancient Egypt and the Indus Valley. Glass beads, vessels, and figurines remained luxury items until the Romans invented the technique of glassblowing in the first century CE. As blowing technology spread, glassware became cheaper and faster to produce in a greater variety of shapes. Items manufactured in the Roman Empire included jars for cosmetics, jugs for condiments, and cups for wine.
So many examples of Roman glassware have survived that Guidetti and her collaborators—which included researchers from the Centre for Cultural Heritage Technology in Italy—could estimate the date of the Aquileia shard by comparing its chemical composition to those of other finds. Its high magnesium and titanium content suggested that the principal ingredient, sodium-rich sand, came from Egypt and that the glass was made between 100 BCE and 100 CE. The substrate’s dark green color is original and arose from the glassmaker’s use of vegetable ash as a reducing agent. The blue and gold colors, however, arose later during the degradation process.
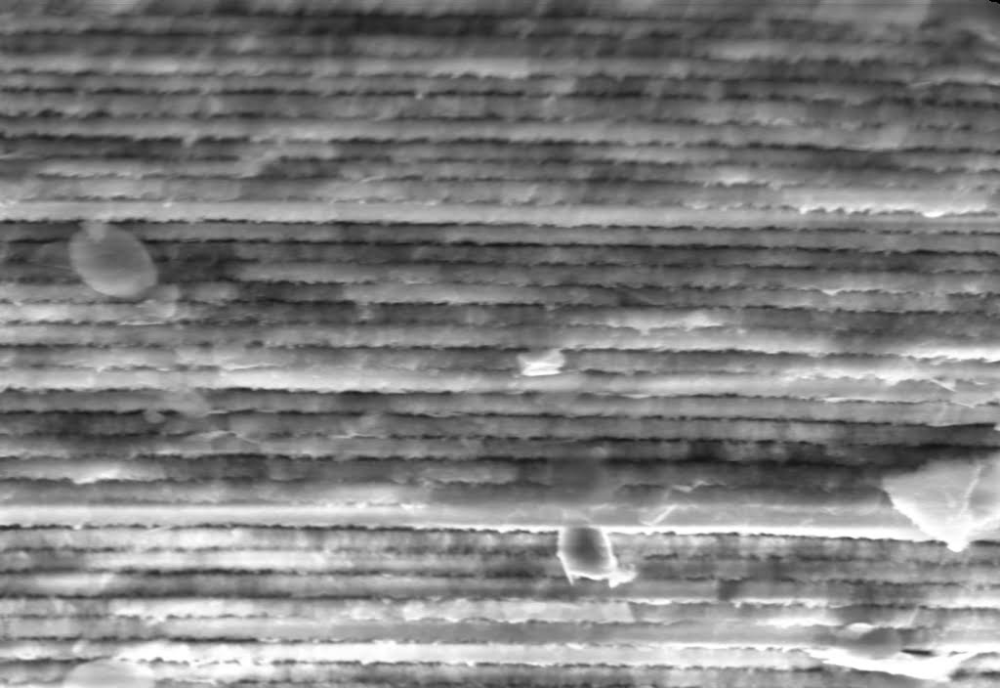
To understand the origin of these colors, Guidetti and her collaborators examined the shard with optical and electron microscopes, discovering structures on several length scales. At the largest scale are micrometer-wide concave domains that are randomly distributed over the surface, like craters on the Moon. Parallel to the surface are thousands of thin layers, mostly made of silica, that alternate in density—and therefore refractive index—between high and low. The thickness of the layers decreases from 320 to 90 nm as the distance from the outer surface increases.
The shard’s layered structure resembles that of an artificial photonic crystal. In a photonic crystal the refractive index is engineered to vary periodically on a length scale comparable to the wavelength of light—that is, a few hundred nanometers. The periodicity creates interference effects that cancel some wavelengths from being reflected back out of the crystal. With one or more of its components blocked, white light that enters a photonic crystal acquires color on its way out.
Similar interference effects produce the colors of the shard. The shiny gold parts of the shard’s surface owe their reflectivity and color to the outermost layers, whereas the deep blue parts owe their color to the innermost layers and to the loss of the outermost layers. The interference effects depend not only on wavelength but also the angle at which light reflects off the surface, with the result that the observed hue changes with viewing angle. This so-called iridescence effect is familiar from butterfly wings and bird feathers (see Arts & Culture: Going Big with Butterfly Wings).
How did such a complicated structure come about? A crucial factor is the pH of the soil that surrounded the shard for the past two millennia. Glass resists acids, but when it’s subjected to highly alkaline solutions, the hydroxyl ions react with the glass’s silicon atoms, dissolving the surface to form pits. The dissolution also occurs when the solution is mildly alkaline, but it proceeds slowly enough that silicon and oxygen atoms have the chance to reprecipitate to form nanoparticles.
Just how those nanoparticles assemble into layers was elucidated in 2021 by Olivier Schalm of the University of Antwerp, Belgium, and colleagues [2]. Their crucial insight was to identify the role of a chemical feedback loop that alters the local pH of the soil at the reaction front. The local pH cycles between high and low values, leaving in its wake alternating layers of loosely packed and densely packed silica nanoparticles.
Because the formation of the thin layers, or “nanolamellae,” on the Aquileia shard was driven by their surroundings, their final structure suggests they grew slowly and steadily without interruption for many centuries. When Guidetti first observed the myriad layers, she was mesmerized by their beauty and regularity. “Finding so many nanolamellae, so regular, and so nicely packed is something that you don’t see on your average photonic sample, let alone in a natural photonic one. It felt like a discovery!”
–Charles Day
Charles Day is a Senior Editor for Physics Magazine.
References
- G. Guidetti et al., “Photonic crystals built by time in ancient Roman glass,” Proc. Natl. Acad. Sci. U.S.A. 120 (2023).
- O. Schalm et al., “Some critical observations about the degradation of glass: The formation of lamellae explained,” J. Non-Cryst. Solids 569, 120984 (2021).