Nuclear Ground State Has Molecule-Like Structure
Nuclei are traditionally described as spherical blobs without much internal structure, a picture that was supported by the classic shell model. For decades, however, researchers have known that protons and neutrons in some excited nuclei form molecule-like structures in which clusters of nucleons play the roles of atoms. Now experiments by Pengjie Li of the Chinese Academy of Sciences and his colleagues provide the clearest evidence to date that nuclei can “clusterize” even in their ground states [1].
Experimental evidence for clusterization was mainly for nuclei with equal numbers of protons and neutrons. These nuclei can partition into superstable alpha particles, which are made of two protons and two neutrons. Adding neutrons changes things, and theory and experiments indicated that neutron-rich nuclei can form clusters in their ground states. The stable isotope beryllium-9, for example, has four protons and five neutrons—the ingredients for two alphas plus an extra neutron. Although experiments with beryllium-9 suggested ground-state clustering, the odd number of neutrons made a theoretical treatment more challenging, and this picture has not yet been verified with state-of-the-art calculations.
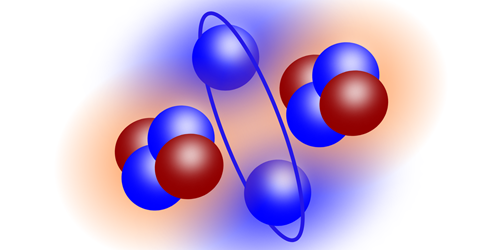
Beryllium-10 is unstable but easier to model, so Li and his colleagues sent a beam of the isotope into a solid hydrogen target, causing hydrogen nuclei (protons) to “knock out” alphas from beryllium-10 nuclei. Detecting all three products—the proton, the alpha, and the remaining helium-6 fragment—of this interaction allowed the researchers to directly measure the locations of the alphas in the original nucleus and compare the results with up-to-date calculations. The team showed that the ground state of beryllium-10 is analogous to a diatomic molecule, with two alpha particles acting like atoms and two neutrons orbiting like a pair of electrons forming a covalent bond.
–David Ehrenstein
David Ehrenstein is a Senior Editor for Physics Magazine.
References
- P. J. Li et al., “Validation of the 10Be ground-state molecular structure using 10Be(p,p𝛼)6He triple differential reaction cross-section measurements,” Phys. Rev. Lett. 131, 212501 (2023).