Five New Isotopes Is Just the Beginning
While some element species (isotopes) can live for hours, weeks, or even millennia, others flash in an out of the world so quickly that scientists have been unable to confirm they exist. Despite the fleeting lifetimes, astrophysicists think these particles, collectively known as rare isotopes, play an important role in the evolution of stars and in the formation of the Universe’s heavy elements. Now researchers at the Facility for Rare Isotope Beams (FRIB) at Michigan State University have made and cataloged five never-before-seen rare isotopes that all contain high numbers of neutrons [1].
This achievement came months after the facility opened its doors and was completed while the equipment operated at a fraction of its full isotope-finding potential, leading FRIB scientists to speculate that these discoveries are just the beginning of multitudes to come. “We proved that we could find new rare isotopes and in less than one year of operations,” says Alexandra Gade, the scientific director of FRIB and a cospokesperson for this isotope-finding study. “That shows a lot of potential for future experiments.”
Each element in the periodic table is listed with its atomic number (the number of protons it contains) and its atomic mass number (the proton number plus the average neutron number). The average neutron number is based on the stable isotopes found on Earth, but an element’s unstable isotopes can have a wide range of neutron numbers. For example, oxygen, which the periodic table lists as containing 8 protons and 8 neutrons, can contain as few as 3 neutrons or as many as 20. The unstable isotopes break apart after varying lengths of time: oxygen-28, for example, decays in a trillionth of a billionth of a second, transforming into oxygen-24, another unstable oxygen isotope, which then also decays, this time in 77 thousandths of a second.
Isotope-generating experiments typically involve smashing atoms into targets and searching the debris for interesting junk. Most of the isotopes created this way contain smaller numbers of neutrons and protons than the original atoms. But researchers are interested in studying very heavy isotopes with proton and neutron numbers that are very close to—and sometimes exceed—that of the original atoms. Such isotopes are thought to play a role in the so-called r process that forms heavy elements in stars and in stellar explosions. But understanding that role is challenging, as these heavy isotopes have tiny probabilities of appearing in Earth-bound experiments. FRIB was designed to meet this challenge by using powerful beams and highly sensitive detection methods.
The experiments performed by Gade and her colleagues involved smashing a high-energy beam of platinum-198 particles into a circular slab of carbon. The fragments of the collisions were then directed into the Advanced Rare Isotope Separator (ARIS) where they were cataloged. ARIS uses a combination of various magnetic and physical mechanisms to filter nuclei by their mass. “You can think of it as a very advanced mass spectrometer,” says Brad Sherrill, who heads the ARIS instrument team. In fact, it is so advanced, Sherrill says, that researchers can separate out a single isotope from a starting collection of 1018 nuclei.
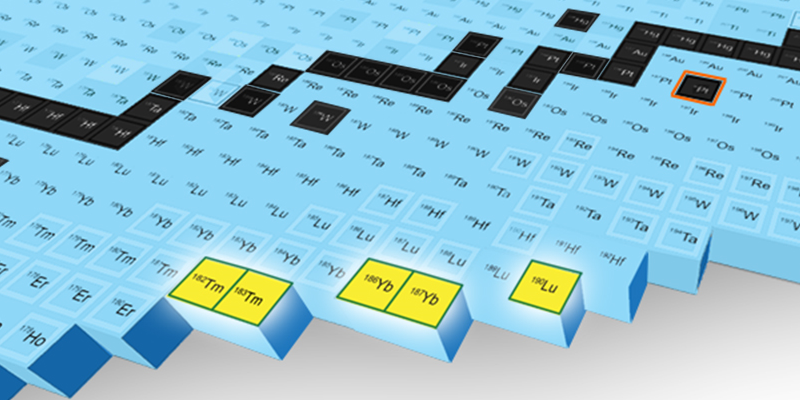
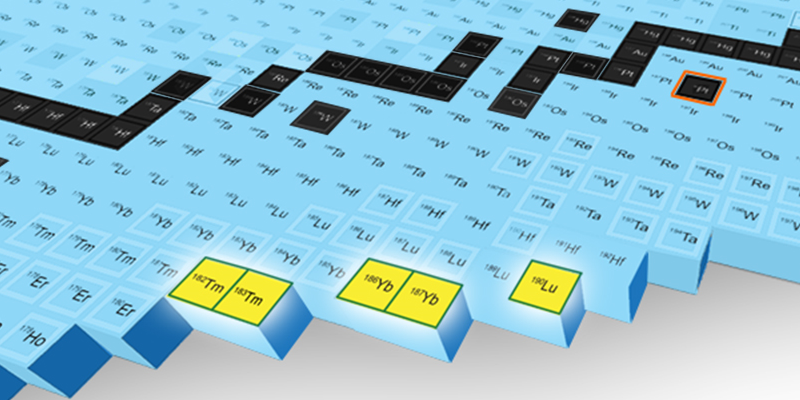
Scouring through the isotopes ARIS detected during an experiment performed in January of 2023, Gade, Sherrill, and their colleagues spotted between 3 and 29 hits from each of five previously undetected neutron-rich isotopes: thulium-182, thulium-183, ytterbium-186, ytterbium-187, and lutetium-190. The team also spotted a single event consistent with the properties of an isotope containing two more neutrons than the 120 of the platinum particles in the beam (the five confirmed isotopes all had less). “Picking up neutrons” in a collision is highly unlikely, but it is a type of event that FRIB researchers are hoping to see more often as the experiment continues. “Some of the new things we saw have extremely low probabilities of being made. But they were still made often enough that over the course of a few days of the experiment we were able to see them,” Sherill says.
“One of the fundamental observables of nuclear physics is whether a given nucleus exists or whether it simply falls apart into its constituents,” says Dan Bardayan the director of the Nuclear Science Laboratory at the University of Notre Dame, Indiana. He notes that FRIB was built to probe the limits of nuclear existence, which is critical to understand if scientists are to correctly interpret reams of multimessenger astrophysical data now being collected by observatories around the world. Jianjun He, an experimental nuclear physicist at Beijing Normal University, agrees. In finding new isotopes, He says that FRIB researchers demonstrate “the impressive capabilities of the facility” and its “great future discovery potential.”
The detections of the thulium, ytterbium, and lutetium isotopes were made when running the platinum beam at 1/270th of the facility’s full capabilities. As the researchers ramp up the flux—the number of platinum particles hitting the target—they will get more beam–target collisions and “more stuff coming out the other side,” Gade says. “We will get orders of magnitudes more of these specific isotopes—and of other rare ones.”
A rare isotope that Gade hopes to spot during the flux ramp-up is one containing 126 neutrons. For neutrons, 126 is a so-called magic number because isotopes containing 126 neutrons are much more stable than those with a few more or a few less. The characteristics of the r-process change around magic numbers, so astrophysicists would like to better understand how these isotopes behave, Gade says. “We have already increased the flux of the beam by a factor of 6, and we have another increase planned soon, so we are well on the path to seeing such an isotope.”
–Katherine Wright
Katherine Wright is the Deputy Editor of Physics Magazine.
References
- O. B. Tarasov et al., “Observation of new isotopes in the fragmentation of 198Pt at FRIB,” Phys. Rev. Lett. 132, 072501 (2024).