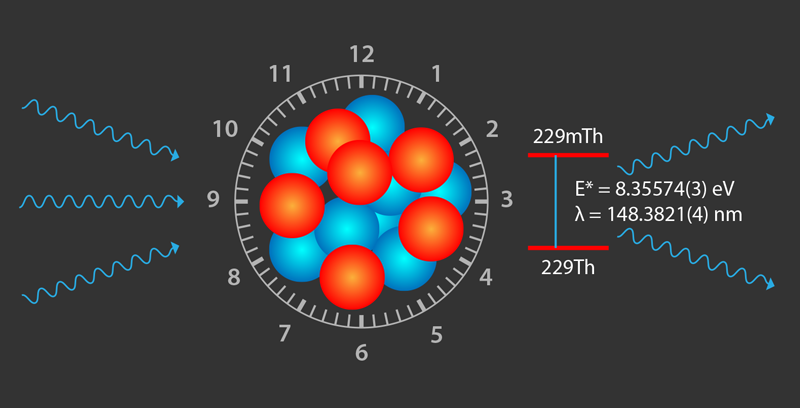
Shedding Light on the Thorium-229 Nuclear Clock Isomer
Any reliably produced, periodic phenomenon—from the swing of a pendulum to the vibrations of a single atom—can form the basis of a clock. Today’s most precise timekeeping is based on extremely narrow electronic transitions in atoms, which resonate at optical frequencies. These stupendously precise optical atomic clocks lose just 1 second (s) in about 30 billion years. However, they could potentially be outperformed by a nuclear clock, which would instead “tick” to the resonant frequency of a transition that occurs in the atomic nucleus instead of in the electronic shell. The most promising candidate for this nuclear standard is an exceptionally low-energy and long-lived excited state, or isomer, of the isotope thorium-229 (229Th). Researchers have now achieved the long-sought goal of exciting this transition with ultraviolet light. Using a laser designed in house, Johannes Tiedau and his colleagues from the German Metrology National Institute (PTB) and the Technical University of Vienna have excited the thorium isomer 229mTh and measured its transition energy and wavelength with unprecedented accuracy, opening the door for realizing a nuclear clock (Fig.1) [1].
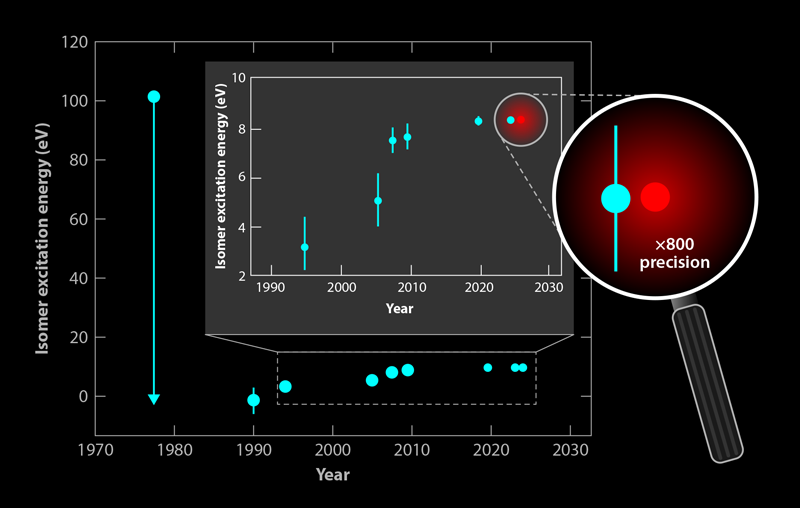
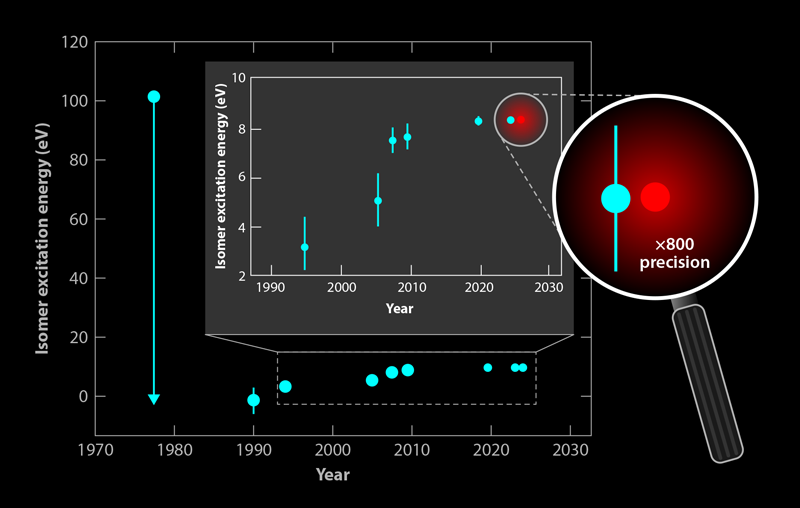
Because an atom’s nucleus is about 5 orders of magnitude smaller than the atom itself, a nuclear frequency standard offers the prospect of reduced vulnerability to distortions by external fields. An ultraprecise and stable “nuclear clock” based on a long-lived isomer is highly attractive [2]. Following the conjectured existence and likely excitation energy of isomer 229mTh in 1976, its uniquely low energy ( ∼8.4 eV) and its long half-life motivated nuclear physicists to learn the details of this exotic nuclear state [3]. For almost 50 years, research toward the realization of a thorium nuclear clock was driven by nuclear spectroscopic techniques, and enormous progress was achieved particularly in recent years [4, 5]. Until now, studies have relied on the excitation of 229mTh entirely via radioactive decays, such as beta or alpha decay of other actinide elements, due to the still large uncertainty regarding its precise excitation energy. Consequently, accessing its resonant excitation from the nuclear ground state using laser light—a step required for the controlled operation of a nuclear clock that’s vital for transitioning to a “laser-dominated” era of nuclear clock research—remained out of reach for decades. Getting there appeared extremely challenging in view of the many (10–12) orders of magnitude to be bridged between the best nuclear precision (8.338 ± 0.024 eV) so far and the optical precision that will ultimately be required for a nuclear clock—that is, precision in the hertz range [6].
This long-awaited breakthrough has finally happened [1]. To achieve it, the researchers first created a medium that could host the nuclei at a high density, where they would be accessible to the laser beam that would excite them. The Vienna team grew heavily Th4+ doped, transparent CaF2 crystals with a 229Th concentration up to 5 × 1018/cm3. Because CaF2 crystals have a band gap larger than the isomer’s excitation energy, they should in principle prevent the isomer’s deexcitation via interaction with its electron shell. A recent experiment at CERN’s ISOLDE facility, which achieved the first observation of the 229mTh radiative decay in CaF2, demonstrated this to be the case: it fluoresced in the vacuum-ultraviolet (VUV) spectral range that corresponds to an approximate excitation energy of 8.4 eV, a sevenfold improvement in precision compared to previous measurements (Fig. 2) [6].
To excite 229mTh, Tiedau and his colleagues first had to develop a broadband, tabletop VUV laser system at the right wavelength (148 nm). While presently no continuous-wave laser exists for 148-nm light, the researchers generated it using a nonlinear optics technique that “mixes” existing laser wavelengths to produce new ones. This process resulted in VUV laser light with a measured spectral linewidth of ≤10 GHz, which is sufficiently broad to perform a search over a wide energy range with a manageable number of measurements. They also had to cool the CaF2 crystal to ≤180 K by placing it on a cold plate in the vacuum chamber where it was exposed to the VUV laser light, to avoid optically damaging it.
Finally, the researchers could precisely measure the excitation energy of the doped CaF2 crystal. They used a photomultiplier tube to collect, focus, and detect their crystal’s fluorescence as they gradually stepped the laser’s wavelength from 148.2 to 150.3 nm. From 20 measurement cycles, each with 50 frequency steps, they observed a distinct fluorescence peak around 148.38 nm from two differently doped CaF2 crystals. A control measurement on a crystal doped with a different isotope, 232Th, emitted no fluorescence signal. The observed central wavelength of the nuclear transition amounted to 148.3821(5) nm, equivalent to a transition energy of 8.35574(3) eV, thus consistent with the 1 𝜎-uncertainty of the value reported in radiative-decay experiments but with 800-fold improved precision [6]. In addition, measurements of the fluorescence decay time revealed an overall radiative lifetime of 229mTh embedded in the CaF2 crystal matrix of 630(15) s. This corresponds to a half-life of the thorium isomer in vacuum of 1740(50) s, consistent with earlier findings and theoretical expectations that take into account polarization effects in the crystal. These effects reduce the isomer’s half-life with the inverse third power of the refractive index.
The new results mark a pivotal point toward the realization of a first nuclear clock prototype. Now that broadband optical excitation of the thorium isomer has been demonstrated, the next goal will be its excitation with a suitable laser featuring a narrow linewidth of a kilohertz or even less. This means that to operate an ultraprecise nuclear clock, the precision of the laser frequency that controls the clock transition has to be improved by at least 6 orders of magnitude. Doing so will be difficult: currently, the only option for narrowband optical control of 229mTh is a VUV frequency comb or a laser source with spectra consisting of 105 equidistant lines that enable exceptionally precise spectroscopic measurements. Ultimately, a narrowband continuous-wave VUV laser has to be developed to efficiently drive a nuclear clock. Such a nuclear frequency standard will, on the one hand, be a superb timekeeper with the potential of an up to tenfold improvement of the accuracy compared to today’s best atomic clocks [7]. On the other hand, unlike atomic clocks, it will also be a novel type of quantum sensor because it is sensitive to phenomena involving the strong interaction. With nuclear clocks, researchers will be able to search for fundamental physics phenomena beyond the standard model of particle physics. A thorium nuclear clock could search with unprecedented sensitivity for predicted temporal variations of fundamental constants like the fine-structure constant [8, 9] or act as a detector for ultralight dark matter candidates [10].
References
- J. Tiedau et al., “Laser excitation of the Th-229 nucleus,” Phys. Rev. Lett. 132, 182501 (2024).
- E. Peik and C. Tamm, “Nuclear laser spectroscopy of the 3.5 eV transition in Th-229,” Europhys. Lett. 61, 181 (2003).
- L. A. Kroger and C. W. Reich, “Features of the low-energy level scheme of 229Th as observed in the 𝛼-decay of 233U,” Nucl. Phys. A 259, 29 (1976).
- P. G. Thirolf et al., “The 229-thorium isomer: Doorway to the road from the atomic clock to the nuclear clock,” J. Phys. B: At. Mol. Opt. Phys. 52, 203001 (2019).
- K. Beeks et al., “The thorium-229 low-energy isomer and the nuclear clock,” Nat. Rev. Phys. 3, 238 (2021).
- S. Kraemer et al., “Observation of the radiative decay of the 229Th nuclear clock isomer,” Nature 617, 706 (2023).
- C. J. Campbell et al., “Single-ion nuclear clock for metrology at the 19th decimal place,” Phys. Rev. Lett. 108, 120802 (2012).
- P. Fadeev et al., “Sensitivity of 229Th nuclear clock transition to variation of the fine-structure constant,” Phys. Rev. A 102, 052833 (2020).
- P. G. Thirolf et al., “Improving our knowledge on the 229mThorium isomer: Toward a test bench for time variations of fundamental constants,” Ann. Phys. 531 (2019).
- E Peik et al., “Nuclear clocks for testing fundamental physics,” Quantum Sci. Technol. 6, 034002 (2021).