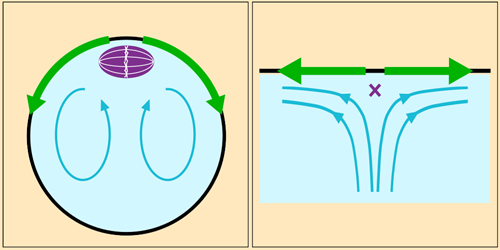
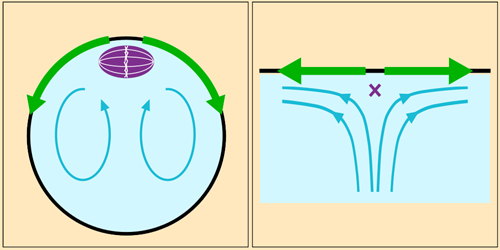
Fluid Flow Anchors Intracellular Structure
In organisms that reproduce sexually, each reproductive cell must contain half of the individual’s complete genetic material. In mammalian egg cells, this halving occurs when the chromosomes are pulled apart by a freely floating protein scaffold called the meiotic spindle. Experiments have shown that this division is preceded by a toroidal circulation in the cell’s cytoplasm, leaving researchers puzzled by how the spindle stays in place. Weida Liao and Eric Lauga at the University of Cambridge have now discovered a mechanism that could be responsible [1].
Cytoplasm circulation is driven by the wave-like motion of polymeric filaments that sprout from one end of the cell. Fluid drawn toward this protein cap constitutes the central part of the toroidal flow. When it reaches the spindle, it spreads out along the inner surface of the cell membrane before returning via the cell’s interior. The spindle sits just below the protein cap, where the flow diverges—a configuration that researchers would expect to become unstable if fluctuations were to move the spindle just slightly off-center.
Liao and Lauga built numerical and analytical fluid dynamics models incorporating measurements gathered from experiments. They found that the spindle’s position would indeed be unstable if the spindle were small relative to the size of the fast-moving part of the flow near the cap. However, the spindle is large enough that a pressure differential induced across it by the flow sucks the structure back into position whenever it’s perturbed. Next, the researchers hope to investigate the flow’s effect on other structures in the cell.
–Marric Stephens
Marric Stephens is a Corresponding Editor for Physics Magazine based in Bristol, UK.
References
- W. Liao and E. Lauga, “Hydrodynamic mechanism for stable spindle positioning in meiosis II oocytes,” PRX Life 2, 043003 (2024).