Molecules on a string
Over recent decades, atomic and molecular physics has blossomed, with much of the work springing from new techniques to translationally manipulate—especially to slow and trap—gaseous atoms and molecules. The ensuing study of slow (cold) atoms and molecules has led researchers into uncharted territories—not just of atomic and molecular physics, but of physics and even chemistry at large. Achieving manipulation on the atomic or molecular level requires some hook by which a magnetic or electric field can seize the atoms or molecules. The role of such a hook is played by the magnetic or electric dipole moment, whose magnitude determines how strong the pull of a given field will be.
In order for a molecular magnetic dipole moment to be sizable (on the order of 1 Bohr magneton), the molecule must be in an open-shell state with nonzero electronic angular momentum. The ground states of radicals (that is, species with unpaired electrons, which makes them more than willing to undergo chemical reactions) all fall into this category and thus exhibit paramagnetism. Most molecular electronically excited states are paramagnetic, for the same reason that the ground states are not, since excitation typically entails raising angular momentum. To give a sense of scale, the interaction energy of a molecular magnet carrying a dipole moment of 1 Bohr magneton with a magnetic field of 1 tesla is 0.67K. This so-called Zeeman energy thus represents only about 0.2% of thermal energy ( 300K). Molecular electric dipole moments, which result from differences in electronegativity of the constituent atoms (that is, their ability to attract electrons), are typically on the order of a debye. In order for such a dipole moment to have an interaction energy (Stark energy) equal to the Zeeman energy of 0.67K, the electric field strength must be 28kV/cm. Since laboratory electrostatic fields are limited to about four times as much, the Stark energy of a polar molecule falls far short of the thermal energy, to only about a 1% level. As Stephen Hogan, Christian Seiler, and Frédéric Merkt from the ETH Zurich show in a paper in Physical Review Letters [1], something can be done about this: the Stark energy of a molecule can be boosted by exciting it to a highly polar Rydberg state in which an electron is put in high orbit. After the manner of a puppeteer, an electric field can then pull on the molecule’s Rydberg electron, like pulling on a string, with the result of manipulating the molecule to and holding it at standstill. The idea of Rydberg-Stark manipulation was originally proposed by Wing [2] and by Breeden and Metcalf [3] in the early 1980s, and its implementation has been a recurring daydream in the atomic, molecular, and optical physics community ever since.
A Rydberg state comes about when a molecule’s electron is promoted to an orbital with a high principal quantum number n (for a review, see Ref. [4]). The spatial separation of the electron in such an orbital with respect to the molecule’s ionic core is large, and thus the concomitant dipole moment is also large, even if the ground-state molecule is nonpolar. For instance, the (nonpolar) H2 molecule in its n=30 Rydberg state is endowed with a dipole moment of 3000 debye, more than 2000-fold that of ammonia in its ground state. As a result, a modest electrostatic field of about 4kV/cm has a grip on H2(n=30) that amounts to the full 300K thermal energy.
The large dipole moments make Rydberg states susceptible to field ionization, which puts a limit on the maximum field strength that can be applied to manipulate them. On the other hand, field ionization can be used to sensitively detect the Rydberg molecules. The large dipole moments also shorten the radiative lifetimes of Rydberg molecules. Fortunately, not all Rydberg states are created equal, and one can pick a state whose decay is more forbidden than that of the others. In particular, Rydberg states with nonpenetrating orbitals are long-lived, often with a lifetime in excess of tens of microseconds, since their overlap with the core wave functions, which drives relaxation, is limited.
Over the last decade, ammonia and other polar molecules were successfully slowed down by Stark deceleration and subsequently loaded into electrostatic/dynamic traps or storage rings [5]. Stark deceleration relies on a loss of kinetic energy, which, by virtue of energy conservation, is brought about by a coerced gain of Stark potential energy in an inhomogeneous electric field. For instance, ammonia molecules with a forward translational energy of about 100K were brought to a standstill by being forced to successively climb about 100 Stark energy hills, generated by switching electric fields between zero and about 100kV/cm. With Rydberg molecules, thanks to their giant electric dipole moments, the same feat can be achieved by just a single deceleration stage (no switching involved) generated by a modest electric field of just a few kV/cm. In principle, this approach is applicable to any Rydberg molecule (or atom) in the gas phase, provided their lifetime is long enough. Rydberg-Stark manipulation was first demonstrated in 2001 by Townsend et al. [6] at the University of Oxford, who observed a sizable deflection of Kr ( n=16– 19) atoms in a dipole electrostatic field.
The story Hogan et al. tell contains intriguing detail about both the dynamics of Stark manipulation and the spectroscopy of the Rydberg states involved. Among the challenges of the experiment was the excitation of the molecular hydrogen’s Rydberg electron to a nonpenetrating orbital, to ensure a long lifetime of the Rydberg state, needed for deceleration, trapping, and detection. In other words, the authors had first to map out the Rydberg states of H2 and their Stark effect so that suitable Rydberg manifolds—such that have little overlap with others—could be identified. The manifolds they settled on, with n=22– 30, were then accessed by a combination of three circularly polarized laser beams acting on a pulsed supersonic beam of H2 and spanning the VUV(105nm), visible ( 550nm), and IR(840nm) domains. The H2 beam was inversely seeded in Kr so that the most probable velocity of H2 was 500m/s (corresponding to a forward translational energy of about 30K). The use of circular polarization ensured the formation of properly oriented f-type orbitals, which are nonpenetrating. The Rydberg spectra were recorded by detecting the ion signal generated from the molecules by pulsed field ionization as a function of the tunable frequency of the IR laser.
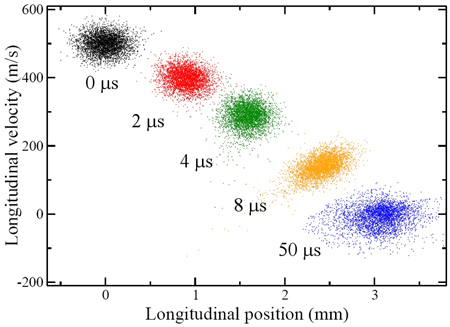
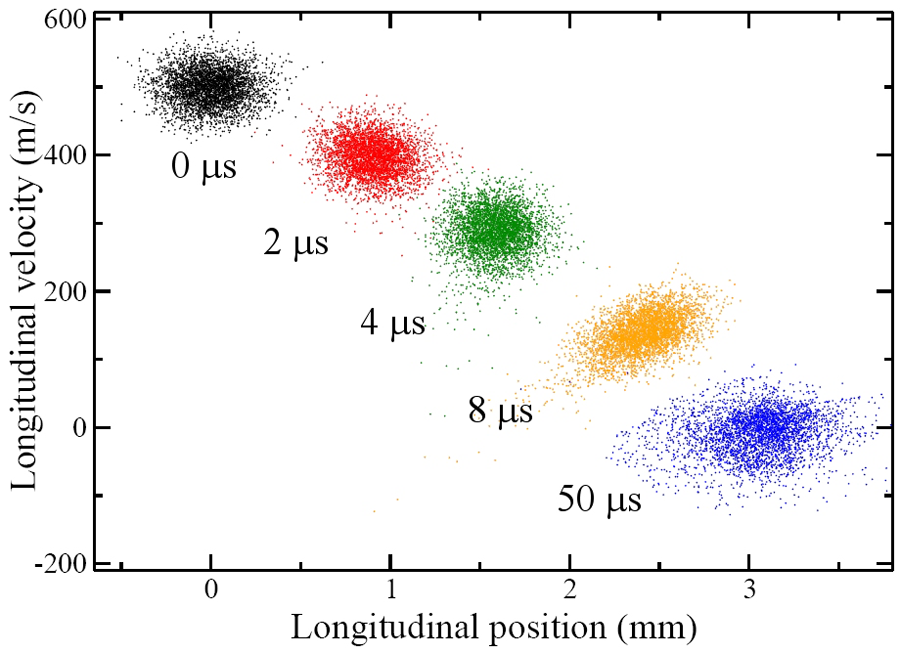
On their flight towards the detection region, the Rydberg molecules were subjected to a fast decaying electric-field pulse, which furnished the necessary inhomogeneous field without causing premature field ionization: ramping down the field maintained the force induced by the field gradient acting on the forward-moving molecules but prevented the molecules from exposure to an ionizing field strength. This was a key enabling trick. The set of six electrodes used for the preparation and deceleration of the Rydberg molecules was then re-energized to a configuration that gave rise to a Stark trapping potential (a minimum of electric field strength in free space, yielding trapping action for low-field seekers). Since both the deceleration and trapping procedures depend on the magnitude of the electric dipole moment, H2 molecules in a range of n states could be loaded and trapped. This was because the dipole moment is proportional to the product nk, where k, on the order of n, depends on the “eccentricity” of the Rydberg orbital. The “acceptance” of the Stark decelerator and trap differs for different Rydberg states, as larger fields afford deceleration and trapping of Rydberg states with a lower dipole moment (lower value of nk). The authors estimate that about 60% of the decelerated molecules could be loaded into the trap, at a density of 107– 108 molecules per cm3 and at a temperature of about 150mK (see Fig. 1). The trap lifetime was measured to be about 50μs, chiefly limited by collisions with background gas, but clearly long enough to afford extracting a plethora of clear and revealing data about both the states involved and their trap dynamics.
In their earlier work, the ETH group joined forces with the group at Oxford to Stark- decelerate molecular hydrogen, tapping a different Rydberg manifold [7]. Subsequently, the ETH group demonstrated Stark deceleration and trapping of Rydberg hydrogen atoms [8]. Achieving now the same feat with hydrogen molecules is particularly significant, given the versatility of the Rydberg-Stark technique and the otherwise limited means available to slow and trap nonpolar and nonmagnetic molecules.
References
- S. D. Hogan, C. Seiler, and F. Merkt, Phys. Rev. Lett. 103, 123001 (2009)
- W. H. Wing, Phys. Rev. Lett. 45, 631 (1980)
- T. Breeden and H. Metcalf, Phys. Rev. Lett. 47, 1726 (1981)
- F. Merkt, Annu. Rev. Phys. Chem. 48, 675 (1997)
- S. Y. T. van de Meerakker, H. L. Bethlem, and G. Meijer, in Cold Molecules: Theory, Experiment, Applications, edited by R. V. Krems, W. C. Stwalley, and B. Friedrich (CRC/Taylor and Francis, Boca Raton, 2009), pp. 509-554[Amazon][WorldCat]
- D. Townsend, A. L. Goodgame, S. R. Procter, S. R. Mackenzie, and T. P. Softley, J. Phys. B 34, 439 (2001)
- Y. Yamakita, S. R. Procter, A. L. Goodgame, T. P. Softley, and F. Merkt, J. Chem. Phys. 121, 1419 (2004)
- S. D. Hogan and F. Merkt, Phys. Rev. Lett. 100, 043001 (2008)