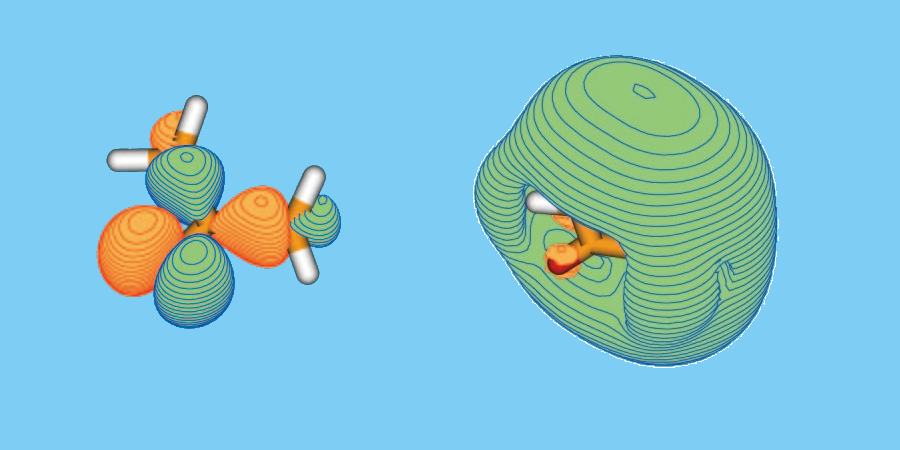
Catching a Molecule in an Excited State
Atoms and molecules in short-lived, excited states often initiate chemical reactions, and now a team has taken a step toward imaging the electronic orbitals in one of these states. They adapted a technique in which probe electrons are scattered off molecules to reveal the energies and momenta of molecular electrons. The researchers examined an excited state of acetone lasting only picoseconds. Improvements in the technology should eventually allow researchers to make 3D images of excited molecular orbitals and could lead to insights into the electron dynamics of chemical reactions.
Understanding the behavior of electronic orbitals—especially the outer, most reactive ones—is key to understanding chemical reactions. In electron momentum spectroscopy (EMS), which was developed in the late 1960s and early 1970s, a continuous beam of electrons is shot at a stream of gaseous atoms or molecules. To create an image of their outer orbitals, researchers measure the energy and momentum of each scattered electron and of each electron ejected from the targeted atoms or molecules.
The problem is that many excited states that are essential to chemical reactions decay within picoseconds, whereas traditional EMS lacks time resolution. Some progress has been achieved using beams of laser-excited atoms [1], but this has not been possible for molecules. And the experiments could not look at the time evolution of the excited states. In 2013, however, Masahiko Takahashi and colleagues at Tohoku University in Sendai, Japan, introduced time-resolved EMS, in which they replaced the continuous electron beam with a pulsed beam [2]. In that initial experiment, the researchers looked at neon atoms in their ground state to test their apparatus. Now, in their latest work, the team has demonstrated the technique’s potential for mapping excited states.
The researchers focused femtosecond laser pulses onto a beam of deuterated acetone, a three-carbon organic molecule with the usual hydrogen atoms replaced by deuterium. First, laser photons excited acetone electrons into the so-called Rydberg state, which has a larger orbital. Then, immediately after excitation, an electron pulse hit the molecules, ejecting excited electrons. The researchers measured the scattered and ejected electrons from both excited and unexcited acetone. By looking at the difference, they calculated the energies and momenta of electrons in the state, finding that their measured values were consistent with theoretical models. For example, the spectrum for the binding energy of electrons in the state showed a peak at 3.5 electron volts, which agrees with predictions for the orbital of the excited state.
Next, the researchers introduced a time delay of 100 picoseconds (ps) between laser and electron pulses. Once excited to the state, the acetone molecule breaks apart into three pieces in about , so after , the spectrum is effectively that of the molecule’s dissociation products. Here too, the results were consistent with theory, confirming the researchers’ hypothesis that they have detected the excited state.
The results have large uncertainties in energy and momentum, and the uncertainty in time ( ) is longer than the state’s lifetime. So the data cannot yet be converted into an image of the excited orbital. However, the team says that relatively simple improvements should boost the precision quite dramatically. For example, the addition of a high-power laser and a pulsed gas nozzle would increase the number of collisions by 300 times, reducing the error bars. “We want to conduct experiments with much smaller intervals of time delay, enabling us to observe how molecular orbitals change during chemical reactions,” says Takahashi. Producing such a molecular orbital movie is an important goal for those studying chemical reaction dynamics.
“This is a breakthrough experiment, and now one has to improve the apparatus and the technique,” says Erich Weigold of the Australian National University, one of the original pioneers of EMS. “But it’s a very difficult experiment, and they should be applauded for their success.”
This research is published in Physical Review Letters.
–Tim Wogan
Tim Wogan is a freelance science writer in London.
References
- Y. Zheng, I. E. McCarthy, E. Weigold, and D. Zhang, “Direct Observation of the Momentun-Density Profile of Excited and Oriented Sodium Atoms,” Phys. Rev. Lett. 64, 1358 (1990)
- M. Yamazaki, Y. Kasai, K. Oishi, H. Nakazawa, and M. Takahashi, “Development of an (,) Electron Momentum Spectroscopy Apparatus Using an Ultrashort Pulsed Electron Gun,” Rev. Sci. Instrum. 84, 063105 (2013)