Orbital Engineering, By Design
Oxides containing transition metals have many fascinating properties, such as superconductivity, ferroelectricity, and forms of magnetism that are linked to the crystal structure. These properties depend on the charge and spin of the transition metal ion and which of its orbitals are occupied by electrons. Tuning these degrees of freedom, however, requires exquisite control over the material’s composition and structure. Ankit Disa and colleagues at Yale University have now demonstrated the ability to selectively populate a particular orbital state of the nickelate LaNiO by sandwiching a thin film of the material between different oxides in a superlattice [1]. The nickelate’s orbital polarization—the ratio of the electron occupancy on two different valence orbitals—is higher than its value for bulk LaNiO , the largest change obtained in a nickelate. Orbital occupancy is directly tied to band structure, so such “orbital engineering” could provide a path to controlling a material’s electronic, magnetic and optical properties. For example, control over the orbital energies and electron occupancies in certain nickel-containing oxides can be a way to make spin switches, strong and inexpensive magnets, and optical devices.
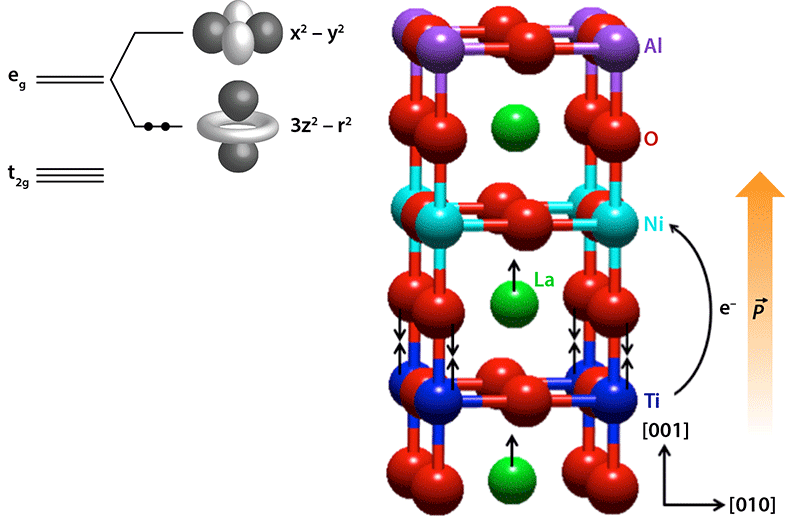
Most attempts to control which orbitals on a transition metal are occupied by electrons have used strain, which can be induced by growing a film of the oxide on a substrate with the same crystal symmetry but slightly different lattice constants. Disa et al. have taken a different approach, designing a heterostructure that modifies both the symmetry at the Ni site and the Ni cation’s valence state. Using molecular-beam epitaxy, a technique that deposits material one atomic layer at a time, they prepared superlattices built from stacks of units consisting of LaNiO (LNO) sandwiched between the oxides LaTiO (LTO) and LaAlO (LAO). The working superlattice was [(LTO) (LNO) (LAO) ] , with the subscripts inside the brackets indicating the number of unit cells of each oxide.
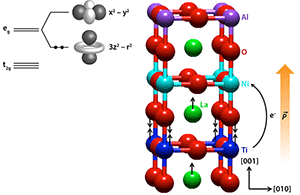
Ti and Ni cations have more than one possible oxidation state and can thus exchange charge. In LNO, both cations are in an octahedral “cage” of O atoms. The negatively charged oxygens create a crystal field that splits the energies associated with the five electronic orbitals on the Ti and Ni sites. They split into three low-lying orbitals (the orbitals) and two higher-energy orbitals (the orbitals). Ni has seven valence electrons and Ti has one, thus they exhibit electron configurations of ( ) ( ) and ( ) ( ) , respectively.
In bulk LNO, the two Ni orbitals, called and because of their shapes, have the same energy. But in the superlattices prepared by the authors, the Ni cation within the LNO unit cell loses its mirror symmetry along the direction perpendicular to the interface (the [ ] direction). As the authors have shown with density-functional-theory calculations, the lower symmetry of the Ni site in the superlattice breaks the degeneracy of the orbitals, causing the orbital to lie higher in energy than the orbital (Fig. 1, inset). Moreover, they predict that it is energetically favorable for the single electron on the Ti cation to move across the interface to the Ni cation, leading to Ti and Ni and a doubly occupied Ni orbital. This predicted charge transfer is born out by x-ray absorption spectroscopy that is sensitive to the Ti valence state. Charge transfer of this magnitude is expected to result in a substantial dipole field, which causes structural distortions at the LNO/LTO interface along the [ ] direction—an effect the authors observed with surface-sensitive x-ray diffraction (Fig. 1).
With two electrons in the -derived band of Ni , the cation develops a significant orbital polarization, meaning the orbital is preferentially occupied over the orbital. To see this, the researchers performed x-ray absorption spectroscopy sensitive to the Ni valence states and compared the spectra obtained with the x-ray electric field parallel and perpendicular to the plane of the interface. Differences in the intensities of these two spectra allowed them to see a different occupancy of the Ni and -derived bands, and to directly determine , the ratio of the number of holes (missing electrons) in the two bands. In bulk LNO, in which there is complete degeneracy in the orbitals, , by definition. In the LNO/LTO/LAO superlattice, , which is approximately half the bulk value.
Researchers have attempted to achieve this extent of orbital polarization using a supporting lattice for the LNO with slightly different lattice constants: the lattice mismatch stretches the material in the plane of the film, which breaks the degeneracy of the orbitals. However, impractically large epitaxial strains ( ) are required to achieve the necessary orbital polarization [2]. Another aspect of this work that sets it apart is the excellent agreement between theory and experiment: the measured dipole moment and the extent of orbital polarization resulting from the superlattice match well with the values predicted by DFT calculations.
Controlling material processes at the level of electrons was listed as one of five “grand challenges” for science in a 2007 report by the U.S. Department of Energy [3] If matter can be controlled at this level, where quantum phenomena rule, then new, highly efficient technologies ranging from artificial photosynthesis to quantum computation could be possible. Yet, many papers claiming to achieve this goal in oxide heterostructures reported effects that weren’t clearly tied to the claimed structure of the material (i.e., the role of unintended defects could not be discounted). As Disa et al. have shown, careful characterization is essential to understand these very delicate cause-and-effect relationships between structure and material properties.
The authors predict that insulators other than LAO could be even more effective at changing the local structural environment of the Ni cations and, therefore, their orbital state. A logical next step would be to test this idea experimentally, although doing so will be difficult because some of the materials predicted to be useful would be rather difficult to deposit with molecular-beam epitaxy. A longer-term goal is to use this approach to engineer orbitals in other transition-metal oxides. Success with different materials would fill the “oxide tool box” with an array of tools for building new, highly functional materials and devices.
This research is published in Physical Review Letters.
References
- Ankit S. Disa, Divine P. Kumah, Andrei Malashevich, Hanghui Chen, Dario A. Arena, Eliot D. Specht, Sohrab Ismail-Beigi, F. J. Walker, and Charles H. Ahn, “Orbital Engineering in Symmetry-Breaking Polar Heterostructures,” Phys. Rev. Lett. 114, 026801 (2015)
- M. Wu et al., “Strain and Composition Dependence of Orbital Polarization in Nickel Oxide Superlattices,” Phys. Rev. B 88, 125124 (2013)
- Directing Matter and Energy: Five Challenges for Science and the Imagination (A Report from the Basic Energy Sciences Advisory Committee), http://science.energy.gov/bes/news-and-resources/reports/20.