Atom Wire Resists Conventions
As the electronic circuits on chips continue to be miniaturized, physicists have naturally looked ahead to the smallest wires possible: those made of only a few atoms or molecules. Researchers have made some rudimentary versions in the lab, but the electrical properties of atomic wires are not well understood. Calculations published in the 19 October PRL show that even in a simple example, those properties can be surprising. While a normal wire increases its resistance with increasing length, they found that the resistance of a chain of carbon atoms oscillates with length, becoming higher for an even number of atoms than for an odd number. The results show that the connections at either end of the atom wire have important effects on the wire’s properties and must be studied in more detail before the technology can be implemented.
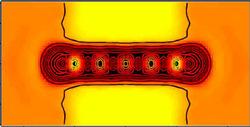
Atom wires are not only small; their lack of impurities should allow them to carry thousands of times the current density that normal copper wires can handle, according to Phaedon Avouris of the IBM Watson Research Center in Yorktown Heights, NY. He also sees atom wires as model systems for learning about carbon nanotubes, the molecular cousins of buckyballs that many researchers see as today’s most practical nanoscale wires. To better understand the properties of these tiny conductors, Avouris and his IBM colleague Norton Lang analyzed a wire made of between three and seven carbon atoms attached to large chunks of metal at each end, which represented connections to a macroscopic circuit. Assuming 0.01 volts were applied across the wire, they calculated its conductance (inverse of resistance).
According to their calculations, the conductance of such a wire does not change continuously with length, but is higher for odd numbers of atoms than for even numbers. The reason, they found, is that for three, five, or seven carbon atoms, there are more available states for electrons to occupy as they traverse the wire. This pattern is determined not only by the structure of the free carbon chain, but also by the number of extra electrons that are permanently drawn onto the wire from the metal contacts. Lang and Avouris also looked at the wire’s conductance as the two electrodes are moved apart, keeping the wire fixed in length and centered between them. Again, the result was surprising: The conductance drops, then increases to a maximum with increasing distance, even as the electrodes’ contact with the wire worsens.
Both results point to the importance of the carbon-metal interactions, says Uzi Landman, of the Georgia Institute of Technology in Atlanta. “It’s not enough anymore to just study the nanowire itself because everything can change when you make the contact.” He says researchers have suspected the importance of metal contacts with carbon-based wires, but “nobody actually did a hard calculation.” Landman says the work should inspire other theorists to perform more detailed calculations of these effects and experimentalists to test the predictions.|


