Hard as a Diamond?
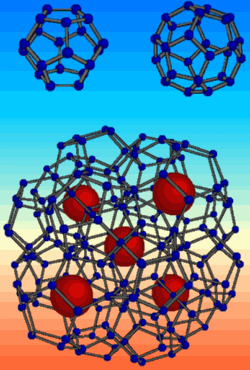
Diamonds are almost as popular in industry as they are with brides-to-be. The hardest material on Earth is great for drills and other machine tools, but it’s expensive to manufacture or mine. Evidence in the 20 December PRL suggests that a form of carbon made from fused spheres of 20 and 28 carbon atoms–relatives of the famous “buckyballs”–could be almost as hard. Since this form of carbon has not yet been synthesized, the team put the equivalent form of silicon under extreme pressure and found it almost as tough as the “diamond” form of silicon, which is structurally identical to real diamond. The experiments also imply that this obscure form of silicon is surprisingly stable under pressure.
One form of silicon clathrate is made from combinations of fullerene-type spherical cages of 20 and 28 silicon atoms each. Although the structure is complex and difficult to visualize, from the point-of-view of each silicon atom, it’s very close to the silicon (or carbon) diamond structure: Each atom bonds with four neighbors arranged at roughly equal positions on an imaginary sphere around it. “We have a material that is locally similar to the diamond structure,” says Alfonso San Miguel of the University of Lyon in France, “but the architecture is very different.”
San Miguel and his colleagues were interested in silicon clathrate as a model of carbon clathrate, but they also wanted to explore the properties of this very low density form of silicon to learn more about this important element. San Miguel says the material may also be useful as a new semiconductor for electronic devices because of its favorable electronic properties.
The team placed the silicon clathrate powder into a diamond-anvil cell and exposed it to x rays from the LURE synchrotron in Orsay, France. Squeezing the sample with pressures up to 15 GPa (about 150,000 times atmospheric pressure), they produced x-ray diffraction patterns that provided information about the crystal structure. Despite its low density and extremely open atomic structure (there are large voids inside each spherical cage), silicon clathrate remained stable and did not convert to the more space-filling diamond structure under pressure. Instead, it converted to the same high-pressure form of silicon that the diamond structure converts to–at about the same pressure (11 GPa ). The clathrate bulk modulus, a measure of compressibility which is related to hardness, came out to just 8% less than that of the diamond form.
San Miguel and his colleagues calculated that if carbon clathrate could be produced, its bulk modulus would be surpassed only by diamond; and if combined with some impurity atoms to fill in the voids, it might be even harder than diamond. Jean Louis Hodeau of the French National Center for Scientific Research in Grenoble agrees that carbon clathrate is likely to be very hard, and he is optimistic that it can be synthesized, based on his own work creating materials made from covalently bonded carbon fullerenes. “But [now] we have to prove it,” Hodeau adds.


