The Quantum and Classical Sides of a Chemical Reaction
Flasks and hotplates are too crude for ultracold chemists. Instead, they drive forward chemical reactions with lasers, which are used to control the quantum states of individual atoms and molecules at temperatures close to absolute zero. Tanya Zelevinsky and colleagues at Columbia University have now used this precision approach to determine when a simple reaction—the dissociation of a diatomic molecule—should be described quantum mechanically and when it should be described (somewhat) classically.
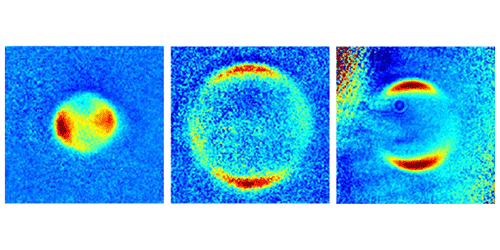
Zelevinsky became interested in the problem three years ago, when her group was using laser light to dissociate two loosely bound ultracold strontium (Sr) atoms. If this diatomic molecule ( ) acts like two classical balls tethered by a spring, then breaking it apart should send the atom fragments flying off in opposite directions. But the group found a more complicated angular distribution, suggesting that the wave nature of the two atoms affected the fragment trajectories.
The team has now perfected their control over the initial quantum state of the molecules, which are chilled to a few microkelvins. They have also carefully studied the fragment patterns over a wide range of dissociation energies, a parameter they control with the laser frequency. Ramping up this energy, they see a crossover in the pattern of fragments, from one that can only be predicted from a quantum model to one where a semiclassical model will do. The crossover occurs roughly where the dissociation energy surpasses the molecule’s rotational energy, and it isn’t sharp. But being able to pin down the transition region could help researchers understand the limits of semiclassical models, which are often used because they are easier to visualize and study than fully quantum models.
This research is published in Physical Review Letters and Physical Review A.
–Jessica Thomas
Jessica Thomas is the Editor of Physics.