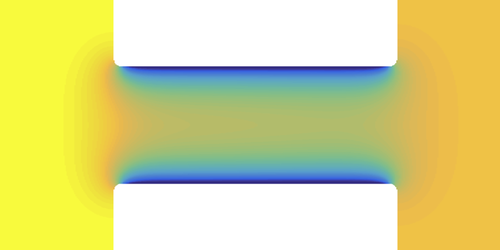
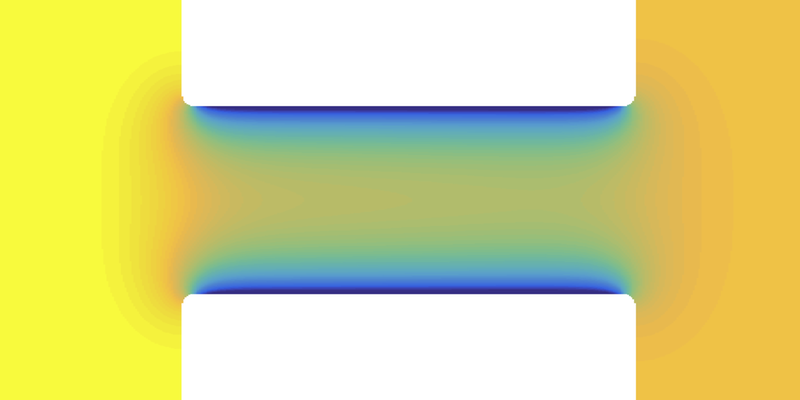
Nanochannel Could Separate Mixed Fluids
Researchers in the Netherlands have proposed a new way of separating fluid mixtures and solutions using porous membranes. They say that the flow rate of a mixture of two fluids through a narrow pore can be used either to partially separate them or to control the transport of dissolved substances through the channel. The idea, still a theoretical proposal, might be useful for separating fluids in industrial processing, or for preventing dissolved material from clogging the pores in membranes.
Porous membranes are widely used in separation processes, for example to filter dissolved or suspended particles from a solvent. But if two fluids are perfectly miscible—they can form a homogeneous mixture together—one might expect the mixture to pass through the membrane unchanged, as long as the pores are significantly wider than the fluid molecules.
However, the composition of a fluid mixture inside a very narrow (nanometer-scale) pore is different from that in the bulk fluid outside it and is not uniform throughout the pore. Thanks to a difference in the strength of the interactions of the components of the mixture with the pore walls, one component may become relatively more concentrated near the walls, a phenomenon known as wetting.
Sela Samin and René van Roij of the University of Utrecht in the Netherlands investigated theoretically how this pore-induced segregation can be altered by the influence of flow through a pore in a way that allows one component to be transported preferentially. They considered two components that are miscible below a “critical temperature” but spontaneously separate into two liquid phases above that temperature.
In general, one of the components—let’s say water, in a mixture of water and some organic liquid—will have a stronger attraction to the pore walls than the other. Water will then be enriched close to the pore surface, forming a wetting layer. If the pore is small enough, this layer will fill most of it, and so there is an enrichment of water inside the pore, relative to the mixture in the “inbound” reservoir.
Can this segregation in the pore somehow be exploited to separate the water from the organic liquid as they are forced under pressure through the pore? Samin and van Roij solved the equations of fluid flow through a cylindrical pore using numerical simulations. They found different types of behavior for different flow rates. At low flow rates, there is steady, preferential flow of water through the pore, at least partially separating the mixture.
For faster flow, the mixture is pushed through the pore unchanged in overall composition: the outflow’s composition is the same as that of the inflow. However, for relatively water-absorbing walls, there is still a column of water-poor fluid in the middle. Dissolved substances that prefer to be in the organic solvent will tend to collect in the center, preventing them from adsorbing on the pore walls and narrowing or clogging the pore. This so-called fouling is a common problem when solutions flow over surfaces or through narrow channels. It occurs in micro- and nanofluidic systems used in chemical and biomedical processing, for example. “We find that the flow could create a self-antifouling effect,” says Samin.
These separation processes should work for a wide range of liquids that have a miscibility critical point, the researchers say. But they work most effectively when the thickness of the wetting layer is large, which occurs close to , where the fluids are on the brink of phase-separating in the bulk mixture.
Other researchers say that, while this approach to separating fluids and solutes shows some promise, it isn’t clear that it will work far from a critical point. “I’m not convinced this phenomenon is not specific to the vicinity of ,” says condensed matter physicist Andrew Parry of Imperial College London. However, Samin says that there is some evidence from computer simulations that, even for a completely miscible mixture such as water and ethanol—comparable to a mixture far from its miscibility critical point—it is possible to get a relatively thick wetting layer [1].
If the approach is more broadly valid, says chemical engineer Abraham Stroock of Cornell University in Ithaca, New York, then it could indeed be useful for separation technology. “The tunability [of the separation] would allow for simpler, cheaper designs of the membrane,” he says, “and one could also potentially use larger pore sizes, providing higher permeability” and faster separation.
This research is published in Physical Review Letters.
–Philip Ball
Philip Ball is a freelance science writer in London. His latest book is How Life Works (Picador, 2024).
References
- S. Gravelle, H. Yoshida, L. Joly, C. Ybert, and L. Bocquet, “Carbon Membranes for Efficient Water-Ethanol Separation,” J. Chem. Phys. 145, 124708 (2016).