Ultracold controlled chemistry
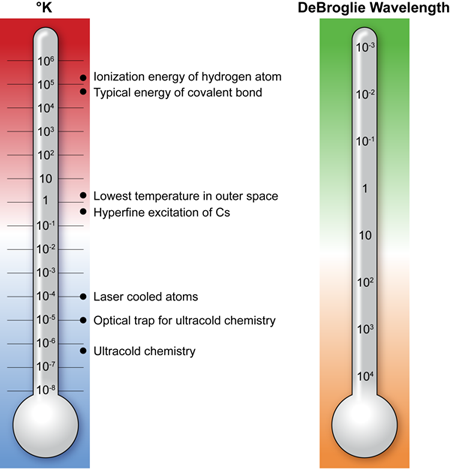
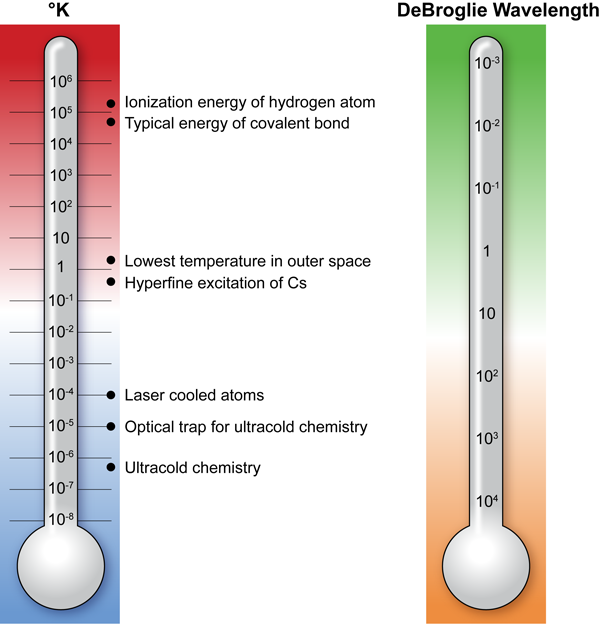
Bound by the Coulomb interaction forces alone, atomic nuclei and electrons combine to form an incredible variety of molecular systems. When molecules react, they undergo chemical transformations leading to rearrangement of atoms within molecules or transfer of atoms between molecules. This process may absorb or release energy; however, the energy change in a chemical reaction is much smaller than the total energy of the Coulomb interaction in a molecule (Fig. 1). This makes chemistry a game of small numbers and a chemical reaction a very complex process to study.
Much of our current understanding of chemical reaction dynamics is due to the development of the technology for producing and colliding molecular beams [1]. A molecular beam is a gaseous ensemble of molecules moving as a whole in the laboratory frame. The molecules are often prepared with a narrow distribution of internal energies (up to a few kelvin) and a low density. When two molecular beams collide, molecules react under single-collision conditions and the reaction products scatter in a particular direction, where they can be detected by a variety of techniques. By varying the angle between the crossed beams, it is possible to tune the collision energy of the molecules. Molecular beam experiments, however, have two significant limitations: they only probe the outcome of a chemical reaction, providing no direct information about the actual process of bond breaking and bond making [2], and, because the density of molecules is usually undetermined, they cannot measure the absolute rate of a chemical reaction. The latter is crucial for calibrating theories of elementary chemical reactions.
Two groups have now taken a completely new approach to study chemical transformations of molecules: Steven Knoop and colleagues at the University of Innsbruck in Austria and the Austrian Academy of Sciences, in collaboration with Jose D’Incao at JILA and Brett Esry at Kansas State University, both in the US, reporting in Physical Review Letters [3], and Silke Ospelkaus and co-workers at JILA and NIST, also in the US [4]. These researchers start with an ensemble of atoms cooled to an ultralow temperature ( ) and confined in an optical potential of a focused laser beam. The atoms are then linked together by a time-varying magnetic field in the work of Knoop et al., or by irradiating the sample of atoms with lasers of two different frequencies in the work of Ospelkaus et al. This produces diatomic molecules with the same temperature as that of the precursor atoms. The atoms and the molecules are trapped in the laboratory frame by the confining potential of the focused laser beam. The trapped molecules are finally allowed to collide with the trapped atoms or with each other and undergo chemical reactions. The collision energy is determined by the temperature of the atom-molecule mixture so these experiments probe chemical reactions at unprecedentedly low temperatures.
The molecules produced by Knoop et al. have unusual properties. They are very extended, with interatomic distances exceeding times the size of the hydrogen atom (Bohr radius) and barely bound, having a binding energy times smaller than the binding energy of the hydrogen molecule in the ground rovibrational state. Their structure is very sensitive to an external magnetic field and their unusual properties lead to unusual chemistry. For example, the authors show that an atom exchange process can occur while all three atoms are separated by large distances of Bohr radii. (In these experiments, and are cesium atoms in different hyperfine levels.) Due to the small binding energy of the molecules, the reaction can be tuned by an external magnetic field from endothermic to exothermic. At ultralow temperatures, this tunes the reaction from allowed to forbidden.
In the experiment of Ospelkaus et al., and atoms are photoassociated to produce molecules in the absolute ground state (i.e., the state of the lowest vibrational, rotational, hyperfine, and Zeeman energy). The molecules are stable in the absence of chemical reaction processes. Chemical reactions release energy and expunge the reaction products from the trap, and so Ospelkaus et al. can measure the reaction rates by monitoring the trap loss in collisions of the molecules with each other or with atoms also prepared in the quantum state of the lowest energy. Because the number of molecules in the trap is known, these measurements yield the absolute rates of the elementary reaction processes. The extremely low temperature of the molecular gas allows for an extremely high resolution of the experiment. For example, the authors measure the reactions of molecules prepared in different magnetic sublevels of different hyperfine energy states. The measurement shows that the reactions can be tuned by transferring molecules from one hyperfine state to another. Collision dynamics of molecules at ultralow temperatures is determined by quantum statistics, and the work of Ospelkaus et al. shows that chemical encounters of fermionic molecules are suppressed. When molecules are prepared in different states, the Fermi suppression is absent and the chemical reactions are dramatically enhanced.
The experiments of Ospelkaus et al. and Knoop et al. mark the advent of ultracold chemistry. The experiments demonstrate that both the internal and external degrees of freedom of molecules at ultralow temperatures can be controlled with high precision, which makes ultracold chemistry controlled chemistry. Ultracold controlled chemistry is unlikely to become a competitive method to synthesize new molecular materials anytime soon; however, the experiments with ultracold molecules offer a new intimate look into the microscopic dynamics of molecules [5,6]. For example, ultracold controlled chemistry offers an opportunity to study a novel class of chemical reactions induced by very weak (fine and hyperfine) interactions. The effect of fine-structure interactions on chemical reactivity has been a long-standing question in physical chemistry [7].
Measurements of chemical reactions at ultralow temperatures can be used to explore the effects of weak long-range intermolecular interactions on chemical dynamics of molecules—another long-standing question in physical chemistry [8]. Ultracold controlled chemistry will provide an ultimate probe of the role of quantum effects in chemistry [9]. Experiments with ultracold molecules can be used to explore chemical dynamics stimulated by many-body quantum effects such as Bose-enhanced chemistry [10] or chemistry in reduced dimensions [11]. At the same time, ultracold controlled chemistry may provide useful information for elucidating chemical dynamics at elevated temperatures. Idziaszek and Julienne [12] have recently shown that the measurements of Ospelkaus et al. [4] can be modeled by a multichannel quantum defect theory with one free parameter that describes the interactions of molecules at short intermolecular separations. This parameter—which can be inferred from an ultracold chemistry measurement—is independent of temperature and encapsulates the dynamics of a chemical reaction. A proper extension of the theory of Idziaszek and Julienne may provide a unique method for mapping intermolecular interactions that govern the dynamics of a chemical reaction at short intermolecular distances. Ultracold controlled chemistry is thus a new regime of chemistry research that will be instrumental for unraveling the complexity of chemical dynamics of molecules.
References
- R. Levine, Molecular reaction dynamics, (Cambridge University Press, London, 2005)[Amazon][WorldCat]
- D. Herschbach, Faraday Discuss. 142, 9 (2009)
- S. Knoop, F. Ferlaino, M. Berninger, M. Mark, H-C. Nägerl, R. Grimm, J. P. D’Incao, and B. D. Esry, Phys. Rev. Lett. 104, 053201 (2010)
- S. Ospelkaus, K.-K. Ni, D. Wang, M. H. G. de Miranda, B. Neyenhuis, G. Quemener, P. S. Julienne, J. L. Bohn, D. S. Jin, J. Ye, arXiv:0912.3854
- R. V. Krems, Phys. Chem. Chem. Phys. 10, 4079 (2008)
- Cold molecules: theory, experiment, applications, edited by R. V. Krems, W. C. Stwalley, and B. Friedrich (CRC Press, Boca Raton, Florida, 2009)[Amazon][WorldCat]
- E. Garand, J. Zhou, D. E. Manolopoulos, M. H. Alexander, and D. M. Neumark, Science 319, 72 (2008)
- D. Skouteris, D. E. Manolopoulos, W. S. Bian, H. J. Werner, L. H. Lai, and K. P. Liu, Science 286, 1713 (1999)
- L. Carr, D. DeMille, R. V. Krems, and J. Ye, New J. Phys. 11, 055049 (2009)
- M. G. Moore and A. Vardi, Phys. Rev. Lett. 88, 160402 (2002)
- Z. Li and R. V. Krems, Phys. Rev. A 79, 050701 (2009)
- Z. Idziaszek and P. S. Julienne, arXiv:0912.0370