Exploring the island of superheavy elements
Over the last thirty years, experimentalists have, so to speak, launched an expedition to explore the predicted “island of superheavy elements”—a region of increasingly stable nuclei around atomic number 114 (Fig. 1). So far they have reported, on average, the discovery of one new element every two and a half years. Now, a Russian-American collaboration headed by Yuri Oganessian at the Joint Institute for Nuclear Research in Dubna, Russia, announces in Physical Review Letters its latest discovery—a new element with atomic number 117 [1]. Two isotopes of the new, still unnamed, element were produced from nuclear fusion reactions from a beam of 48Ca ions that impinged on target nuclei of 249Bk. This discovery fills the gap between elements 116 and 118, so that now elements are continuously known from hydrogen up to element 118, and offers the possibility to study the heaviest known nuclear island of stability with greater detail.
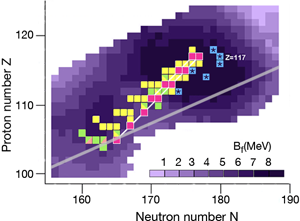
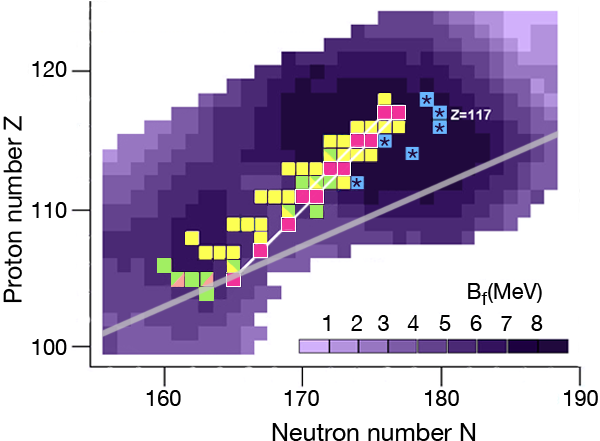
In the region of heavy elements, decay occurs primarily by the emission of α particles, spontaneous fission, and β+ or β- decay. The lifetime of the nuclei and how exactly they decay depends on several factors. The simplest of the nuclear models, which imagines the nucleus as a charged liquid drop, predicts that the most stable ones are located in the “valley of stability,” the boundaries of which are determined by the interplay between nuclear and electric forces. Figure 1 shows a chart of the heaviest elements and their isotopes, where the straight line marks the bottom of the valley of stability in this region. Nuclei along this line are stable against β decay.
The preferred arrangement of protons ( Z) and neutrons ( N) in shells provides a second effect on nuclear stability, which is analogous to the way electrons fill atomic orbitals. Closed shells are energetically favorable, and this modulates the smooth trend of the binding energy predicted by the liquid drop model. There is an especially high binding energy for nuclei with double shell closures, that is, where the neutron and proton shells are both closed. The “shell model” does a good job of explaining the stability of nuclei with neutron and proton shell closures at 2,8,20,28,50, and 82, and when the neutron shell closes at 126. (Helium- 4, which has a double shell closure with 2 neutrons and 2 protons, is an example of a highly stable element.) But where the closed shells are located in the heavy element region is less certain. Various models predict they should occur at Z=114,120, or 126 for the protons and N=172 and 184 for the neutrons.
The nuclear shell model explains why nuclei are spherical at closed shells and deformed in between these cases. The model also predicts the energetic barrier for a particular nucleus to undergo fission [2]. The higher the fission barrier is, the longer the fission lifetime. The highest fission barriers are expected for nuclei just above the closed shell that occurs at Z=114 and just below the closed shell that occurs at N=184 (The darker purple regions in Fig. 1 denote higher fission barriers, outlining the island of stability for the superheavy elements).
What is difficult to calculate, and not included in the calculated barrier shown in Fig. 1, is how the presence of an odd number of protons or neutrons affects fission lifetimes: in lighter nuclei the presence of an odd number of nucleons can hinder fission by a factor of 10 to 104, depending on angular momentum. In the case of odd-odd nuclei, that is, nuclei with an odd number of protons and an odd number of neutrons, the total hindrance factor is the product of the ones of the odd proton and neutron, so that the lifetime could be up to 108 times longer than that of the neighboring even-even nuclei.
During the last ten years, the Flerov Laboratory of Nuclear Reactions in Dubna, Russia, has made considerable progress in laying out new terrain in the superheavy elements—progress that is providing nuclear physicists with the information they need to understand what factors govern stability for these elements. An essential part of their experiments was developing an intensive beam of doubly magic and neutron-rich 48Ca for acceleration at the laboratory’s U400 cyclotron. With this beam, they irradiated several neutron-rich isotopes of actinide targets ( 238U, 242Pu, 244Pu, 243Am, 245Cm, 248Cm, and 249Cf) to form a “compound nucleus.” (The compound nuclei are marked in Fig. 1.) The compound nuclei are hot, having excitation energies of 35– 45MeV and evaporate three to four neutrons. In most of the experiments, the produced evaporation residues—the superheavy nuclei which are eventually measured—were separated from the beam and other reaction products by a gas-filled recoil separator, and position-sensitive silicon detectors identified the isotopes. The team applied a well-established position-and-time correlation analysis—a technique that was developed to identify elements 107 to 112 using 208Pb and 209Bi targets at the GSI SHIP [3]. (Note that the isotopes produced at GSI and the isotope 278113 synthesized recently at RIKEN [4] are not shown in Fig. 1.)
The produced superheavy nuclei, as well as several of their daughter nuclei, are α active so that decay chains of various lengths are measured. All of the chains end by spontaneous fission. The known decay chains (from Ref. [5]) are plotted in Fig. 1, where the two new chains emerging from the isotopes 293117 and 294117 of the new element are highlighted as well. The decay properties of these two new chains give further support to the assignment of the previously identified elements, which were based on cross-bombardments and the measurement of excitation functions. Even more important, the conclusion has to be drawn that the heaviest nuclei are located already quite well inside the island of superheavy nuclei.
By following the decay chains of these superheavy elements, and identifying where they end, researchers can map out the boundaries of the island of stability. A closer examination of the decay chains reveals that all chains from odd-odd nuclei, including the new isotope 294117, are extremely long and end at dubnium ( Z=105) by spontaneous fission or electron capture and subsequent fission of the even-even rutherfordium daughter nucleus. The region of low fission barriers is crossed due to combined fission hindrance of the odd proton and the odd neutron. Chains from even-even nuclei end at isotopes of copernicium ( 112) at the south-west border of the island, where the fission barriers decreased to 6– 7MeV and hindrance factors do not exist. Chains from odd isotopes of even elements end at darmstadtium ( 110), where fission barriers are 1MeV lower, at 5– 6MeV, but hindrance from the odd neutron hampers fast decay. The new chain from the odd element isotope 293117, having an even neutron number, ends already at 281Rg due to less hindrance than in the case of the neighboring chain from the odd-odd 294117.
The decay chains from the new element 117 fit well into the picture of an island of stability created by shell effects. Due to the α-decay chains and the high probability that fission will occur at the end of the chains, the borderline at the south-west of the island can be considered fixed. Now we are faced with the question of how far the island extends in the other directions, and what experimental paths can lead us to these regions. Certainly, fusion studies of heavy ions will continue to look for the north-east and north-west borderlines. However, the longest lifetimes are expected in the south-east near the β-stability line and close to the neutron shell N=184. There, α lifetimes are long and in the case of odd-odd nuclei, spontaneous fission could be so significantly hindered that we might find these nuclei on earth or in meteorites. In the laboratory, we may have a chance using fusion reactions with radioactive beams or nucleon-transfer reactions. Theorists are already working on predictions in this direction [6]. The experimental challenge will be to find the proper beam and target combination, the optimum beam energy, and a highly sensitive detection method.
References
- Y. T. Oganessian et al., Phys. Rev. Lett. 104, 142502 (2010)
- P. Möller et al., Phys. Rev. C 79, 064304 (2009)
- S. Hofmann and G. Münzenberg, Rev. Mod. Phys. 72, 733 (2000)
- K. Morita et al., J. Phys. Soc. Jpn. 73, 2593 (2004)
- Yu. Ts. Oganessian, J. Phys. G 34, R165 (2007)
- V. Zagrebaev and W. Greiner, Phys. Rev. C 78, 034610 (2008)