Are Mammals Ferroelectric?
Ferroelectricity—a spontaneous electrical polarization—was first discovered in potassium sodium tartrate, a salt, in 1920. The effect is reminiscent of ferromagnetism. At first, hydrogen bonding was thought to be essential for this property, until the finding of the ferroelectric oxide BaTiO3 two decades later. In 1974, ferroelectric liquid crystals were produced, following theoretical predictions based on intuitive symmetry arguments. Since then, intense scientific efforts have uncovered hundreds of ferroelectric materials in various forms, including submicron films, nanotubes, nanowires, integrated ferroelectrics, etc., many of which are neither hydrogen bonded nor oxides [1]. Now, writing in Physical Review Letters, Yuanming Liu and co-workers at the University of Washington in Seattle, with collaborators at Boston University, Massachusetts, demonstrate evidence of ferroelectricity in mammalian tissue [2].
The most prominent feature of ferroelectric materials is the ability to reverse their polarization by applying an electric field, again reminiscent of ferromagnets. The reversal typically occurs below a phase transition temperature, leading to a characteristic hysteresis loop of polarization plotted against applied electric field, and a butterfly-shaped loop in the deformation-voltage graph—properties that are the basis of commercial ferroelectric memories. Ferroelectric materials are usually also piezoelectric and pyroelectric, in that their polarization behavior can also be modulated by mechanical forces or temperature, respectively. Such versatile properties, and the ability to control them through several parameters (pressure, temperature, electric field) make them useful in many advanced technologies, such as ultrasound devices, high-quality infrared cameras, sonar, and vibration sensors, to name but a few examples.
Due to its attractive properties and applications, it is tempting to ask if ferroelectricy, like piezoelectricity and pyroelectricity, has also evolved in biological tissues through natural selection. Indeed, piezoelectricity in bone was discovered in 1957 and later reported in other biological materials as diverse as DNA, collagen, and silk. The piezoelectric effect is now generally thought to play an essential role in biological force sensing. For example, sustained applications of an electrical potential could stimulate both resorption and growth of bone [3]. Most recently, piezoelectricity has also been suggested to be one of the toughening mechanisms in seashells, the effect triggering energy dissipation upon external mechanical forces [4]. Analogously, pyroelectricity in bone and tendon was discovered in 1966 [5] and later reported in various biological tissues, including skin epidermis and the spinal cord [6]. The pyroelectric effect can, for example, enhance the efficiency of thermal energy conversion to electrical energy, for example, for sensor applications [6]. In comparison, however, there is so far very limited evidence for ferroelectricity in biological systems, especially in soft tissues.
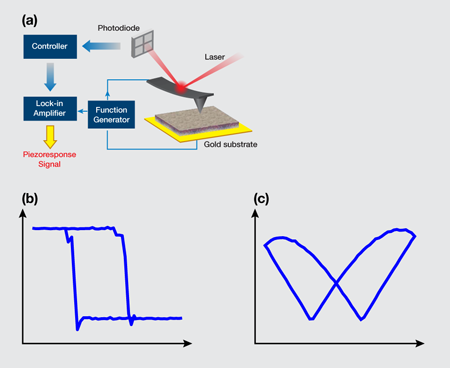
In their pursuit of ferroelectricity in soft biological tissues, Liu et al. [2] performed a study with a piezoresponse force microscope (PFM)—a scanning probe microscope that analyzes the electrical signals generated from the response when the PFM tip is pressed to a surface, thus generating an image of the surface. They used the PFM to probe the inner layer of porcine aortic walls, called “intima,” which consists of a monolayer of cells supported by an internal elastic lamina. The setup is illustrated in Fig. 1(a). Their samples were cut from the aortic wall and then dried out. To increase the sensitivity of the measurement, an ac voltage near the resonant frequency of the cantilever-sample system was applied through the conductive atomic force microscope tip. In this way, both vertical and lateral displacements on the order of 100 picometers (pm) were acquired under a 3 volt (V) ac voltage. To verify if a sample possessed ferroelectricity, the team attempted to switch its polarization by applying a sequence of dc voltages with a triangular sawtooth profile, while a 3 V ac voltage was simultaneously applied to extract the corresponding piezoresponse. To minimize the effects of electrostatic interactions, data were taken during the “off” state at each dc voltage step.
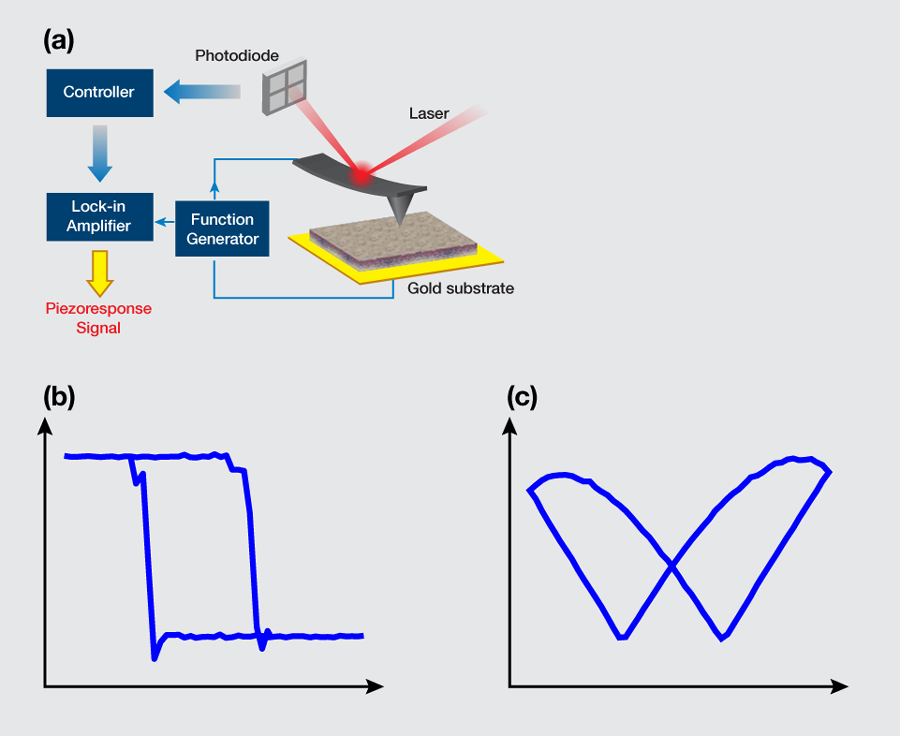
Liu et al. obtained phase-voltage hysteresis loops at different points of their samples [Fig. 1(b)] consistently, indicating an overall ferroelectricity of the sample. The reversal in the piezoresponse phase occurred at a certain voltage, the so-called coercive voltage, which was measured to be approximately 8.4 V on the positive side and -10.8 V on the negative side, the asymmetry reflecting the existence of an internal polarization. The phase contrast was approximately 180 degrees, which is a clear indication of polarization switching. Associated with the phase reversal, the deformation-voltage butterfly loops were also observed [Fig. 1(c)], which saturated at a relatively high voltage, as expected, suggesting that the response was piezoelectric instead of electrostatic. The characteristic hysteresis and butterfly loops shown in Figs. 1(b) and 1(c) establish the existence of ferroelectricity in these samples. Through detailed switching-spectroscopy mapping, it was further recognized that the internal polarization of the aortic walls was biased outward. The relaxation studies also confirmed that the inward polarization switched by a negative voltage is unstable, and would reverse spontaneously to the more stable outward orientation shortly after the switching voltage was removed. Quantitative analysis based on the damped harmonic oscillator model suggested that the piezoelectric coefficient of the aortic wall was on the order of 1 pm/V, which is almost comparable to that of aluminum nitride or gallium nitride thin films [7].
The above discovery poses interesting questions regarding the purpose of ferroelectricity in aorta walls, where the blood pressure is highest and most pulsatile. Could the engineering principles of ferroelectricity, only mastered in modern times by mankind, have already been implemented in nature for millions of years? For example, could ferroelectricity function as a critical component in a local integrated memorylike structure, together with nerves within the aorta? Could it help sense very small temperature changes in our blood flow to help maintain temperature homeostasis? Could it also be a force sensor and play a role in blood pressure homeostasis? Or could it help dissipate the mechanical work into thermal energy when the aorta walls are subjected to strong transient shear flows in the blood? While these questions may stir up curiosity and further investigations from a fundamental standpoint, another important question is, how can we benefit from this finding through engineering? For example, can the change of ferroelectricity due to the local damage in the aorta walls be probed as a damage reporter? Can it guide effective drug delivery to local damaged zones in the aorta, and can ferroelectricity in the aortal walls be manipulated to prevent cholesterol from depositing onto the aorta walls, or help clean the deposited cholesterol, which may also possess ferroelectricity [8]?
Beyond all these possibilities and promises, however, it should be emphasized that how ferroelectricity occurs in such a biological system as aortal walls is still a mystery, although it has also been reported recently in sea shells, so there are precedents for biological ferroelectricity [4]. Interestingly, the intima’s structure, when examined by atomic force microscopy reveals a fibrous network with globular particles. Could these be connected with the observed ferroelectricity? Will the same behaviors exist under normal physiological conditions as well as in dry samples studied in the laboratory?
References
- J. F. Scott, Science 315, 954 (2007)
- Y. Liu, Y. Zhang, M-J. Chow, Q. N. Chen, and J. Li, Phys. Rev. Lett. 108, 078103 (2012)
- S. R. Pollack, E. Korostoff, W. Starkebaum, and W. Lannicone in Electrical Properties of Bone and Cartilage, edited by C. T. Brighton, J. Black, and S. R. Pollack (Grune & Stratton, New York, 1979)[Amazon][WorldCat]
- T. Li and K. Zeng, Acta Mater. 59, 3667 (2011)
- S. B. Lang, Nature 212, 704 (1966)
- H. Athenstaedt, H. Claussen, and D. Schaper, Science 216, 1018 (1982)
- C. M. Lueng, H. L. W. Chan, C. Surya, and C. L. Choy, J. Appl. Phys. 88, 5360 (2000)
- P. Boldrini, J. Theor. Biol. 87, 263 (1980)