Packing Heat to Store Energy
Renewable energy sources like wind and solar produce electricity intermittently, so meeting demand may require storing some of the energy for later use. In Physical Review Letters, a researcher presents an idealized model for a system that would store the electric energy as heat, a technology that is in active development in industrial labs. The theory uses few parameters and shows that the efficiency depends essentially on the storage temperature. The model could guide future design efforts aiming for the most cost-effective energy storage system.
Electric energy storage is one of the major engineering challenges in moving toward an electricity grid based on renewable energy sources that don’t produce electricity continuously. Batteries are too expensive for large-scale storage, so power companies have turned to two technologies: pumped hydro, which requires a high-elevation reservoir of roughly 10 million cubic meters of water; and compressed air, which uses an underground cavern with a volume of around a million cubic meters. These conditions, however, are not likely to be found next door to a wind farm or solar panel array.
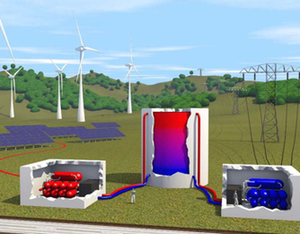
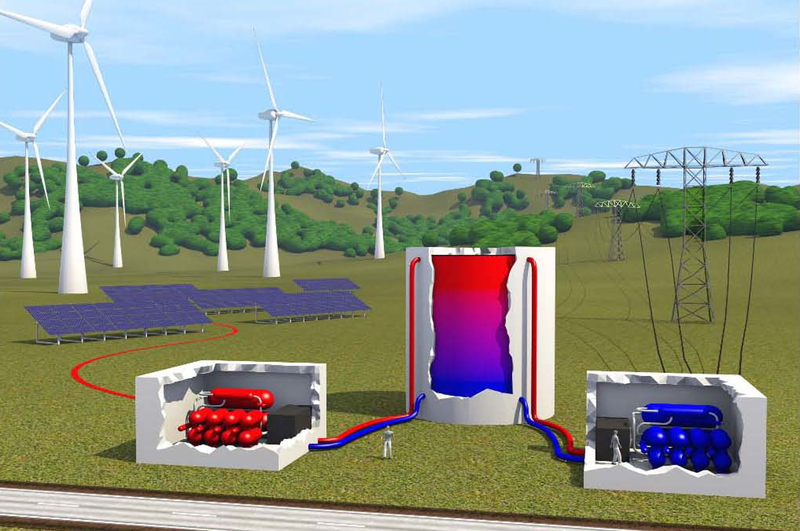
By contrast, storing electric energy as heat in a tank less than cubic meters in volume—a technique called pumped heat electricity storage (PHES)—could be done practically anywhere. To understand PHES, first imagine boiling a tank of water by running electric current through heat-generating wires and then later using the steam to turn a turbine and generate electricity. The problem is that there’s a theoretical limit to a steam engine’s efficiency. A steam engine is one type of heat engine, a machine that converts heat into work, and even if it operated perfectly, one could recover only about of the electricity used to boil the water. “Probably most people who have thought about heat storage have concluded that it’s useless,” says André Thess of Ilmenau University of Technology in Germany.
Thess and others have realized that much higher efficiencies are possible if the electric current powers a heat pump that heats the water, rather than using current to heat it directly. A heat pump moves heat from a colder place to a warmer place, using a fraction of the electricity needed to generate the heat from scratch. (A refrigerator is one type of heat pump.)
Several research groups in the last few years have proposed different PHES systems using a variety of storage materials, such as water, salt, or metal, with different temperature ranges. Predicting the performance of such systems requires some complicated calculations involving many parameters. To fully grasp the potential of PHES and to more easily compare designs, Thess has developed a model of a generic system that can be characterized by a small number of parameters.
He began by assuming that both the heat pump and the heat engine could be represented as idealized devices. A common assumption is that these thermodynamic systems are “reversible,” which means that there is no internal friction, and there are no unrecoverable heat transfers. Such perfect components would give efficiency for the full round trip (electricity-heat-electricity), because the heat pump compensates for the heat engine’s low efficiency. But Thess points out that a reversible PHES system would take an infinitely long time to produce any electricity. A better idealization, he says, is for the heat engine to be optimized for maximum power—producing electricity as rapidly as possible, but with less than perfect efficiency.
Under the maximum power assumption, Thess derived an expression for the round-trip efficiency that depends only on the ratio of the ambient temperature to the storage temperature. For example, a PHES system that heats water from ° to ° Celsius should have an efficiency of around . That’s considerably less than the efficiency of pumped hydro storage, but Thess says that the PHES facility would occupy one tenth of the space. Furthermore, PHES can have higher efficiencies when the storage temperature is increased. A facility that heats aluminum to its melting temperature of ° Celsius could operate at efficiency, he predicts. However, a lower efficiency water-based system might prove more cost-effective when a complete economic analysis is performed.
The use of maximum power thermodynamics is useful but mostly as a “worst case” scenario because of its lower efficiency, says Jonathan Howes, the chief technical officer for Isentropic Ltd., a UK company developing its own PHES system [1]. “For a well-conceived and designed system the efficiency should lie between that of a reversible, perfect system and the [maximum power] result,” Howes says.
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics Magazine based in Lyon, France.
References
- The Isentropic design uses hot and cold reservoirs at ° and ° Celsius. The company claims their round-trip efficiency is between and