A Fragile Union Between Li and He Atoms
Neutral atoms or molecules can enter into three types of unions that vastly differ in the strength of the bond between them. While the binding energy of chemically bound atoms is typically on the order of electron volts (eV), hydrogen bonds are about an order of magnitude or two weaker, and van der Waals forces are weaker still. The last arise because of the mutual attraction of electric dipoles carried by, or induced in, atoms or molecules, and their interaction energy is conveniently measured in kelvins (energy divided by Boltzmann’s constant; there are about 104 kelvins to an eV). The weakest variety of van der Waals forces—dispersion forces—stem from the mutual induced-dipole–induced-dipole attraction that is indispensable for achieving noble gas cluster formation and condensation [1] but is also prominent in condensed matter systems and in physisorption [2].
First invoked in 1930 by Fritz London, dispersion forces are a necessary consequence of the quantum electron correlations that govern the attraction between atoms or nonpolar molecules at large separations. Proportional to polarizability, London dispersion forces are especially weak for He atoms, which shun entering into unions with other like or unlike atoms as a result [3]. At the same time, the tight binding of helium’s electrons, reflected in high excitation energies and hence a puny polarizability (the smallest of all ground-state atoms), makes the distance of closest approach between helium and other atoms as small as the other atom (or molecule) allows. Thus, of all He atom combinations, it is smallest for the He-He pair. As a result, for He2, the London dispersion forces, which vary with the inverse sixth power of the distance, get a chance to loom quite large before being overwhelmed by the repulsive interaction at short range.
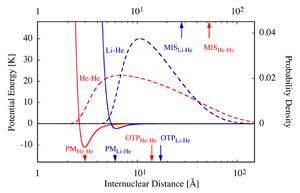
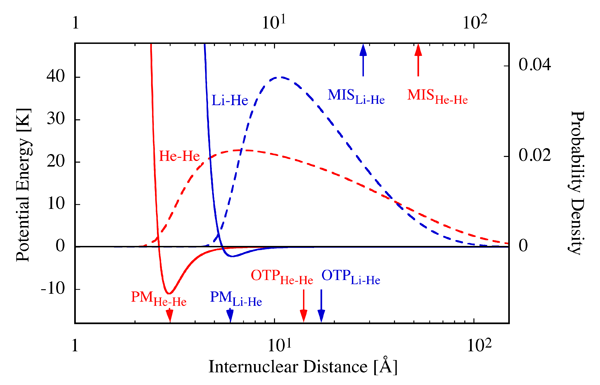
This interplay between attraction and repulsion lends He2 a fragile single bound state whose energy lies just about 1 millikelvin (mK) below the dissociation limit [4] (see Fig. 1, also for an energy conversion factor). No wonder then that it took decades to demonstrate the existence of He2 experimentally. The quest for He2 culminated in the work of Peter Toennies and Wieland Schöllkopf from the Max Planck Institute in Göttingen, who made use of the matter-wave properties of helium clusters to identify He2 via diffraction of a helium molecular beam from a transmission grating [5]. It took nearly two more decades to establish experimentally the existence of a second helium-containing diatomic, LiHe, as Naima Tariq and colleagues from the University of Nevada at Reno (UNR) now report in Physical Review Letters [6].
The experimental work by Naima Tariq et al. breaks new ground in several respects: (i) LiHe was prepared via ternary Li+He+He collisions of Li with and within a cryogenic He buffer gas [7]; (ii) LiHe was detected spectroscopically; (iii) the binding energy of LiHe was determined from the measured dependence of the LiHe density on temperature; (iv) LiHe is paramagnetic; (v) LiHe is polar; (vi) according to theory [3,4], LiHe is bound by a potential whose minimum is about 5 times shallower and its position about 2 times larger than that of the He2 potential (see Fig. 1). Below, I will address these distinctive features of the new work in turn.
Instead of relying on molecular beams, the UNR team has implemented the He buffer-gas cooling technique developed and used previously by John Doyle and co-workers at Harvard to study the formation kinetics of van der Waals complexes [8], an effort that has led to indirect evidence for the AgHe dimer [9] and directly inspired the search for the LiHe dimer.
The UNR apparatus consists of a cryostat-cooled cell filled with He buffer gas whose density and temperature can be varied between 1017– 1018 atoms per cm3 and 1.6– 5.0K, respectively. The He density is sufficiently high to ensure thermalization via elastic collisions of the Li atoms produced by pulsed-laser ablation of lithium metal within the cryogenic cell as well as for driving ternary Li+He+He collisions, necessary for the LiHe formation.
A key ingredient of the UNR team’s work is the spectroscopic detection of the fragile LiHe dimer, which possesses excited electronic states (closely related to those of the Li atom) that can be accessed at optical wavelengths (the transition used for the detection of LiHe occurs at 671 nanometers). Moreover, the position of the last bound vibrational state of the electronically excited state of LiHe that partakes in the optical transition is very weakly bound, which results in a fortuitously strong transition from the ground state (which is also very weakly bound). This, combined with a rapid rate of LiHe formation, affords an abundant fluorescence signal that allowed the UNR experimentalists to obtain a clean high-resolution spectrum. The spectrum revealed that at the temperature of the buffer gas, only a single rovibrational state of LiHe is populated. This state is split, however, into a hyperfine doublet, which arises because of the nuclear spin of the more abundant bosonic isotope of lithium, 7Li, used in the experiment. The populations of the hyperfine-doublet states are found to be roughly statistical.
Having established that only a single rovibrational state of LiHe is populated, the UNR team proceeded to determine the state’s binding energy by measuring the temperature dependence of the optically detected LiHe density. The result is a 7Li4He binding energy of 34±36mK, consistent with the theoretical prediction of about 6mK. The calculated rotational constant [4] of 40mK precludes the possibility that any higher rotational state than the rotationless ground state could be supported by the only bound vibrational state of LiHe, well in keeping with the experimental findings (the next rotational state would have an energy of 80mK).
The LiHe dimer is a radical, thanks to the unpaired electron spin of the Li atom, and therefore subject to a sizable Zeeman effect. It is also heteronuclear and therefore polar, although its electric dipole moment is yet to be determined. Both the Zeeman and Stark effects—and their combination—may reveal additional particulars about LiHe’s structure, dynamics of formation, as well as enable the manipulation of the radical’s translation, including its trapping [10].
One of the most remarkable features of He2 is its size, as given by the mean separation of the two constituent atoms. This amounts to nearly 50 angstroms (Å) [4], making He2 the largest (ground-state) diatomic species. It’s about 70-fold the size of the H2 molecule. The humungous size of He2 is caused by its loose bond and by the small mass of He. Although the outer turning point for the zero-point vibration of the dimer occurs at only 14 Å, the dimer can tunnel its way out through the 1mK barrier imposed by the binding energy. In fact, He2 spends most of its time—about 80%—in the classically forbidden region beyond the confines of its potential, and is thus a prime example of a halo species [11] (see Fig. 1).
What about 7Li4He? Because of its greater binding energy and mass (about 6 and 112 times that of He2, respectively), the mean distance between its constituent atoms is down to about 29 Å [4] (see Fig. 1). It would be down even more, had the position of the minimum of the LiHe potential been the same as for He2. It is, however, shifted out to about 6 Å, twice the He2 value, because of a less-compact binding of Li’s electrons and the concomitant predominance of repulsion at correspondingly higher internuclear separations. At the same time, the enhanced Li polarizability makes the London dispersion forces stronger and thereby affords a potential energy well at large internuclear separations, which is deep enough to bind the dimer. As a result, like with He2, the bond between Li and He keeps the atoms far apart rather than close. They spend most of their time (over 60%) in the far out, classically forbidden halo reaches of the potential, beyond the outer turning point of about 17 Å, where their union is more fragile than elsewhere. The halo behavior is a manifestation of universality [12], as it transcends the specific type of forces involved and can be found, e.g., in nuclear systems.
The ability to produce and detect He dimers within the buffer-gas environment opens the prospect of assembling a whole class of He-containing diatomics and larger clusters, whose properties may be of interest to few- and many-body physics, including the physics of Efimov states [13], which are yet another manifestation of universality. The halo dimers can also be used to study the Casimir retardation effects [14], which arise because of the finite speed of propagation of the electromagnetic field and thereby affect the dispersion forces at large separations. Last but not least, He clusters are of interest to quantum chemistry, whose accuracy is challenged by the fragility of the He bonds.
References
- K. T. Tang and J. P. Toennies, “Johannes Diderik van der Waals: A Pioneer in the Molecular Sciences and Nobel Prize Winner in 1910,” Angew. Chem. Int. Ed. 49, 9574 (2009)
- B. Friedrich, D. Hoffmann, and J. James, “One Hundred Years of the Fritz Haber Institute,” Angew. Chem. Int. Ed. 50, 10022 (2011)
- U. Kleinekathöfer, K. T. Tang, J. P.Toennies, and C. L. Yiu, “Potentials for Some Rare Gas and Alkali-Helium Systems Calculated from the Surface Integral Method,” Chem. Phys. Lett. 249, 257 (1996)
- U. Kleinekathöfer, M. Lewerenz, and M. Mladenovic, “Long Range Binding in Alkali-Helium Pairs,” Phys. Rev. Lett. 83, 4717 (1999)
- W. Schöllkopf and J. P. Toennies, “Nondestructive Mass Selection of Small van der Waals Clusters,” Science 266, 1345 (1966)
- N. Tariq, N. A. Taisan, V. Singh, and J. D. Weinstein, “Spectroscopic Detection of the LiHe Molecule,” Phys. Rev. Lett. 110, 153201 (2013)
- H. Suno and B. D. Esry, ”Three-Body Recombination in Cold Helium-Helium-Alkali-Metal-Atom Collisions,” Phys. Rev. A 80, 062702 (2009)
- N. Brahms, T. Tscherbul, P. Zhang, J. Klos, R. Forrey, Y. S. Au, H. Sadeghpour, A. Dalgarno, J. M. Doyle, and T. Walker, “Formation and Dynamics of van der Waals Molecules in Buffer-Gas Traps,” Phys. Chem. Chem. Phys. 13, 19125 (2011)
- N. Brahms, B. Newman, C. Johnson, T. Greytak, D. Kleppner, and J. Doyle, “Magnetic Trapping of Silver and Copper, and Anomalous Spin Relaxation in the Ag-He System,” Phys. Rev. Lett. 101, 103002 (2008)
- B. Friedrich and J. M. Doyle, “Why are Cold Molecules so Hot?,” Chem. Phys. Chem. 10, 604 (2009)
- A. S. Jensen, K. Riisager, D. V. Fedorov, and E. Garrido, “Structure and Reactions of Quantum Halos,” Rev. Mod. Phys. 76, 215 (2004)
- Ch. H. Greene, “Universal Insights From Few-Body Land,” Phys. Today 63, 40 (2010)
- F. Ferlaino and R. Grimm, “Forty Years of Efimov Physics: How a Bizarre Prediction Turned into a Hot Topic,” Physics 3, 9 (2010)
- S. K. Lamoreaux, “The Casimir Force: Background, Experiments, and Applications,” Rep. Prog. Phys. 68, 201 (2005)