A Quantum Dot Shows Its True Colors
Researchers are developing tiny particles that can glow in a range of colors for use in electronic displays and other devices. They have thought that a promising type of these so-called quantum dots made with manganese could only glow orange, but now a team reports in Physical Review Letters that—based on images of many individual particles—they can shine in a range of hues from green to red. The team says their results may lead to new types of lighting, screen displays, and even solar panels.
Photoluminescent quantum dots are semiconductor nanoparticles less than about 10 nanometers in diameter that can absorb photons of a shorter wavelength (usually ultraviolet) and then emit photons at a longer wavelength (usually visible light). For most quantum dots, their size affects the allowed quantum states for electrons and holes, the charge-carrying particles in the material. These states determine the energies and therefore the colors of the emitted photons. By producing these nanoparticles in a variety of sizes, researchers can make dots that shine in almost any color.
Quantum dots are being developed for new kinds of highly efficient and bright video displays and sources of lighting, for example, by using ultraviolet, light-emitting diodes (LEDs) to excite the dots. Biologists are also using quantum dots for purposes such as revealing the locations in a cell where specific genes are expressed.
Many quantum dots are made with cadmium selenide, but researchers have been exploring semiconductors mixed (or “doped”) with manganese because they are more durable, more efficient, and don’t require cadmium, which is highly toxic. Manganese-doped quantum dots, however, have been thought to fluoresce only in a single shade of orange, limiting their usefulness. Researchers believed that since the fluorescence comes from electrons bound to a single ion, rather than electrons and holes roaming through the entire nanoparticle, the nanoparticle’s size shouldn’t affect the color. Measurements also showed that the orange light represents a relatively broad peak in the spectrum—rather than a more narrow, “pure” spectral line—which isn’t good for displays.
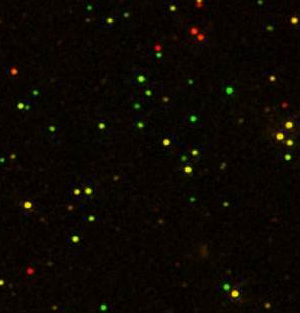
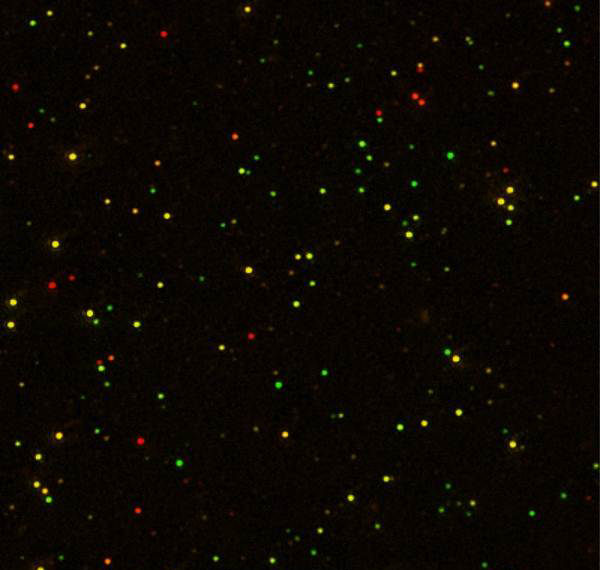
D. D. Sarma of the Indian Institute of Science in Bangalore and his colleagues found some surprises when studying quantum dots made of a zinc-cadmium-sulfur alloy doped with manganese (cadmium isn’t necessary but was used for practical reasons). While previous studies of such quantum dots looked at a solution of millions of particles at once, Sarma and his team poured a solution of the particles over a thin polymer film and observed individual particles with a microscope.
“What we found, to our shock, surprise, and intense excitement, is that each nanoparticle gave a different color,” Sarma says. The researchers recorded the light from more than a thousand particles and analyzed 354 in detail. The glowing particles ranged from deep green to red, and their emission spectra were five times narrower than previously measured. The characteristic broad, orange hue that past researchers had seen was simply a blend of all of the colors from every nanoparticle, says Sarma.
To explain the wide range of colors, the researchers suggest that the energy levels of each manganese ion—which dictate the quantum dot’s color—are influenced by the electric field from the surrounding semiconductor atoms. The atomic arrangement near the surface is different from that near the center, giving rise to differing electric fields, which lead to different manganese energy levels. So a quantum dot’s color depends on whether most of its manganese ions are near the surface or near the center of the nanoparticle.
To test this idea, the team covered manganese-doped nanoparticles with a layer of undoped material, thereby burying any manganese ions that might reside near the surface. These particles emitted slightly bluer light than uncoated ones, as expected based on the researchers’ calculations. By controlling the location of the manganese ions more precisely, researchers could, in principle, tune the color of the dots, the team says.
In addition to uses in lighting and displays, Sarma says researchers are also exploring using manganese-doped quantum dots to boost efficiency in electricity-generating solar cells, exploiting the dots’ ability to convert solar ultraviolet photons into visible photons. Paul O’brien of the University of Manchester in the UK says the new results “extend our perceptions of what can be achieved, which could be of significant practical importance” for device applications.
–Marcus Woo
Marcus Woo is a freelance science writer in the San Francisco Bay Area.