Protein Physics of Pruney Skin
Your skin soaks up water when you bathe, which may partially explain wrinkly fingers. This absorbent property of skin may stem from the unique packing of protein filaments in skin cells, and now two theorists have developed a complete thermodynamic model for the hydration and expansion process, as they report in Physical Review Letters. This reversible process harnesses a balance between filament expansion induced by excess water and contraction driven by a springlike tension as the filaments are stretched, the researchers found. The model’s predictions agree with experimental data, and the findings could help researchers better understand how skin diseases work and help them devise durable or water-absorbent materials.
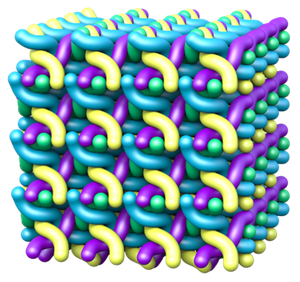
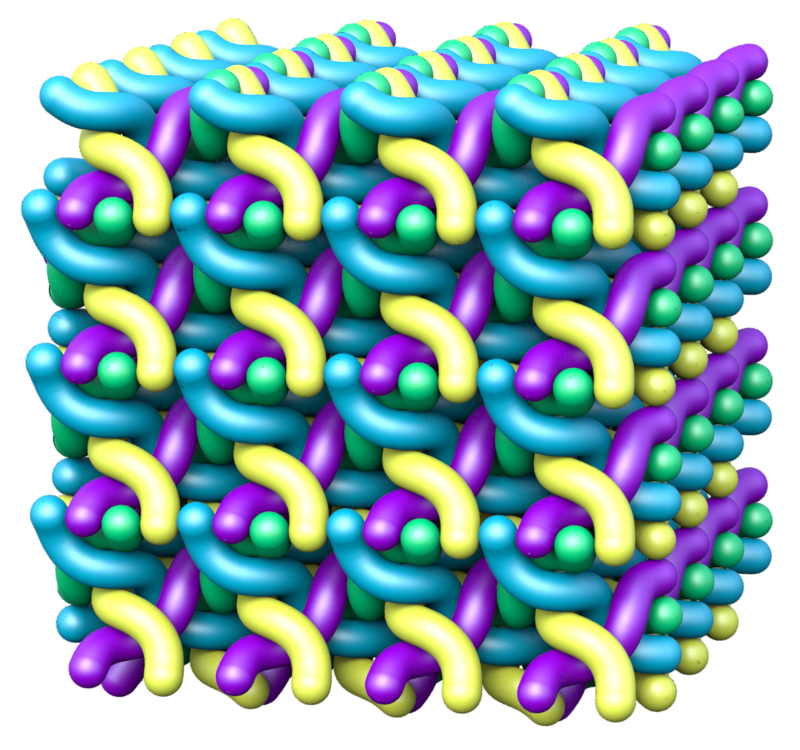
Skin has the remarkable ability to absorb water, increasing its volume substantially and then reverse the process as it dries, without any ill effects. This property serves several functions, one of which is to regulate the level of hydration inside the body. The swelling and absorption of water occur in the outermost skin layer, which is made of dead cells called corneocytes that are stacked in layers like bricks. Corneocytes are filled with a stiff network of filaments made of a form of the protein keratin. In 2011, Myfanwy Evans, then a graduate student at the Australian National University (ANU) in Canberra, working with ANU’s Stephen Hyde, used geometric modeling to propose a structure for these filaments (the so-called sigma-rod packing). In the model, each filament is a long helix pointing in one of four directions in space, and they interpenetrate to form an orderly, three-dimensional lattice. This structure could increase its volume by five times when the helices stretch out [1].
Evans, now at the University of Erlangen, and Roland Roth of the University of Tübingen, both in Germany, have now taken the next step—confirming that the structure actually could help skin cells swell and shrink and learning how the process might work. They developed a thermodynamic model that describes how the system’s energy varies as the network’s spacing and helices’ sizes change. The researchers first calculated the filaments’ solvation free energy—essentially, the system’s willingness to absorb water—as the helices stretch and found that this energy decreases, meaning that the structure is inclined to expand and absorb water.
But some other force must eventually act in opposition to reverse the system’s expansion, Evans and Roth reasoned, since the process reverses easily in real cells. Inspired by previous filament elasticity measurements [2], they realized that the tension in a stretched filament could provide the counteracting force. As with a spring, the more you stretch a filament, the larger the elastic energy. The researchers calculated this energy and added it to the solvation free energy to get the total mechanical energy associated with expansion and contraction.
The team then produced a sort of energy “landscape,” a surface whose height represented energy and whose two horizontal coordinates were the helix spacing (or lattice size) and the helix radius. They identified a range of values where the energy was at or near its minimum, and this “energy valley” turned out to be roughly trough-shaped. Evans and Roth propose that as the filament structure swells and shrinks, it always remains within this valley as it moves between the two extreme states at opposite ends of the trough. The researchers conclude that the keratin filaments’ geometry must be crucial to skin’s response to water because it keeps the system in an energy range that enables but also curbs expansion. The team also predicted the fraction of the volume occupied by keratin (as opposed to empty space or water) at the two extremes and found that it went from 11% to 38%, close to the range of 15– 35% that Evans and Hyde had calculated from experimental data.
This “pioneering work” yields valuable insight into skin function at intermediate scales, between macroscopic and molecular, says Lars Norlén of the Karolinska Institute in Stockholm. He says researchers need this so-called mesoscale information to understand the mechanisms of, and thus devise new treatments for, dermatitis and other skin diseases involving abnormal skin moisture. Researchers could also create cloths or durable building materials that exploit skin’s mesoscale properties, he says.
–Puneet Kollipara
Puneet Kollipara is a freelance science writer in Washington, DC.
References
- M. E. Evans and S. T. Hyde, “From Three-Dimensional Weavings to Swollen Corneocytes,” J. R. Soc., Interface 8, 1274 (2011)
- D. S. Fudge, T. Winegard, R.H. Ewoldt, D. Beriault, L. Szewciw, and G.H. McKinley, “From Ultra-Soft Slime to Hard α-Keratins: The Many Lives of Intermediate Filaments,” Integr. Comp. Biol. 49, 32 (2009)