Snapshots from the 2015 APS March Meeting
Preparing Physics Ph.D.s for Jobs in Industry
Graduate schools groom young talent for a career in academic research. Yet, more than of physics Ph.D.s leave academia, and a significant fraction of them ultimately take jobs in the private sector. A new report from the American Physical Society (APS, the publisher of Physics), says graduate schools need to better prepare physics students with the skills they’ll need for industrial careers, including jobs in finance and for technology companies. The recommendation comes from industrial physicists, who were at the meeting to discuss the report.
Barbara Jones, a scientist at IBM, said that while academic research has become increasingly specialized, the qualities industries value most are breadth and the ability to tackle many types of problems. Team-player spirit is also important, said Steven Lambert, who serves as the APS industrial liaison. The report also stresses the importance of communication skills: Research papers and talks are usually aimed at experts, but in industry, researchers need to explain their work in simple and concise terms to managers and customers. Lambert said that the most effective ways of preparing students would be mentoring from industrial physicists, contact with recent graduates working in industry, and access to internships flexible enough to fit into thesis work.
Materials of the Future
Our ability to master materials and use them in novel ways defines the world we live in—so argued Sharon Glotzer at the Physics-sponsored pizza-and-beer event. What materials will shape the society of tomorrow? Will silicon and plastic still dominate the scene, or will new materials emerge that can respond to the environment, be their own energy sources, self-assemble into specific structures for targeted applications, or self-replicate? While this might sound like science fiction, recent advances in synthesis and fabrication have delivered colloidal systems (solutions containing dispersed nano- to micron-sized particles) with characteristics that could allow smart, programmable materials to become reality.
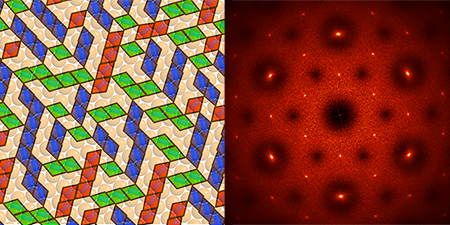
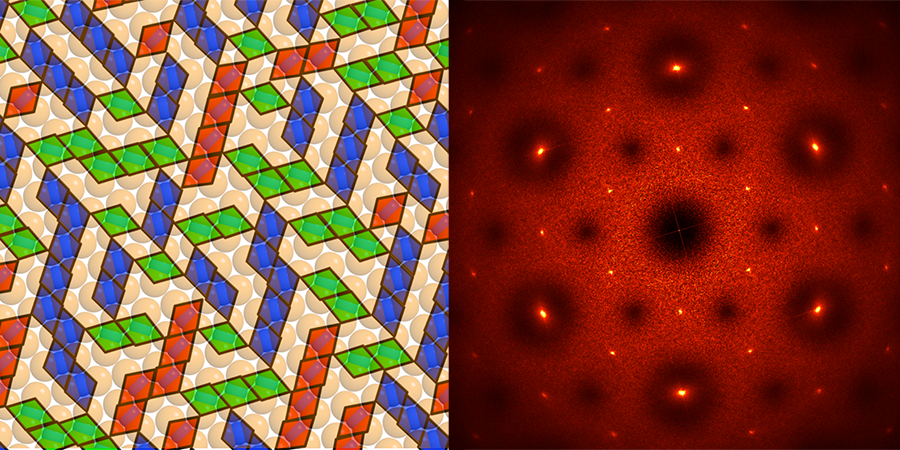
Using theory, simulations, and experiments, Glotzer and her colleagues have demonstrated systems featuring a wide array of capabilities. For example, they engineered colloids that spontaneously assemble into braided fibers that can be stretched or contracted by an applied electric field—behaving much like artificial muscles. Another class of colloids, called “lock-and-key” colloids (see Fig. 1), could provide reconfigurable systems: “lock” and “key” molecules with complementary shapes could bind in various configurations that could be used to encode and store information at very high densities. According to Glotzer, terabytes of information could potentially be stored in only a tablespoon of such colloids. Before these materials can break into everyday applications, Glotzer believes that researchers need a better theoretical framework to describe the thermodynamics, interactions, and energy balance of these extremely complex systems.
Physicists Solve Art Dispute
Art historians have long believed that Pablo Picasso was one of the first artists to use colors made for painting houses, rather than the more expensive oils and acrylics favored by his contemporaries. However, despite some existing clues to the artist’s preferences (including a few photographs showing Picasso with tins of house paint), scholars judged that the evidence was inconclusive.
Thanks to high-energy x rays and a serendipitous find on eBay, Volker Rose at Argonne National Laboratory demonstrated that the white paint in Picasso’s “Still Life with Three Fishes, Moray Eel and Lime on White Ground” came from an enamel house paint, as he reported in a session on “Physics for Everyone.” Rose was able to find on eBay samples of house paint commonly found in France at Picasso’s time. By analyzing the emission spectra of a fleck of white color from the painting and comparing those data with spectra from his eBay samples, Rose showed that both shared the same chemical makeup: mostly pure zinc oxide. Traditional artistic paints, on the other hand, generally contained higher levels of impurities and different white pigments (lead, titanium, and calcium).
Rose and his colleagues are now applying their x-ray techniques to study other works of art. These days, they are analyzing old photographs and 19th century inks to gain insights on the chemistry underlying corrosion and degradation of old documents, and to look for methods to stop or reverse these processes.
Hints to High- Superconductivity from a Thin Material?
Researchers still don’t know why superconductivity occurs in iron-based compounds—materials that, like cuprates, superconduct at a relatively high temperature. Perhaps answers will come from the recent, and surprising, finding that a single unit cell of , grown on the insulator , superconducts at a temperature five times higher than its bulk form does. Speaking at a session on superconductivity in two-dimensional materials, Jian Wang of Peking University gave an overview of his measurements, first reported in 2014, showing that a 0.55-nanometer-thick layer of has an onset of superconductivity around 50 kelvin (K), compared to 8 K for bulk .
A huge and expensive research effort—almost entirely based in China—is underway to make new samples and explain the effect, which was first seen in surface spectroscopy measurements. In November, researchers at Jiao Tong University in Shanghai reported that they had seen the resistivity of on plunge to zero at 109 K. If it can be reproduced by other groups, the result would “change our entire view” of high-temperature superconductivity, said Ivan Bozovic of Brookhaven National Laboratory in a phone call. He said the result is important because the electronic structure of on is so much simpler than it is in bulk iron compounds or in the cuprates. If one mechanism explains all three systems—and Bozovic hopes it does—it would suggest that the complex electronic structure of the cuprates and iron compounds isn’t essential for high-temperature superconductivity. (See this commentary in Nature Physics from Bozovic and Charles Ahn of Yale University).)
–Jessica Thomas and Katherine Wright