Bacteria Stick Together as Living Crystals
A solid crystal is an array of atoms held in a precise geometric arrangement by electromagnetic attraction, but now researchers have discovered a kind of crystal made of living bacteria. The cells are held together by the hydrodynamic suction the bacteria generate with their flagella. The 2D “bacterial crystals” formed on the surface of a glass slide, but the researchers don’t yet know whether such crystals form in the natural world or what function they might serve. The team mathematically modeled the behavior of the crystals and identified three characteristics of the cells that allow them to crystalize.
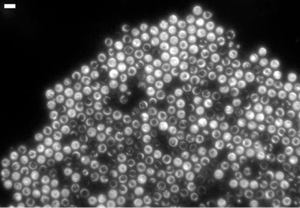
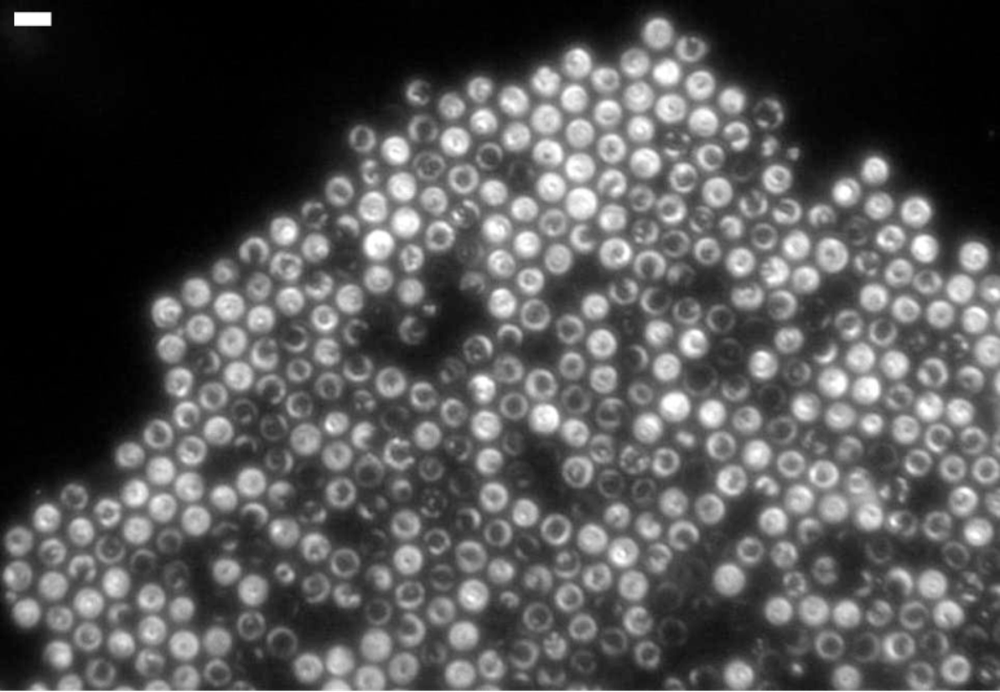
Thiovulum majus are large ( 5- to 20-micrometer-diameter), spherical bacteria that live in salt marshes and survive on oxygen extracted from the water. They are covered with hundreds of tiny flagella that allow the species to swim faster than any other bacteria. The cells rotate as they swim, and they often join together into disordered clumps called veils through which they collectively pump water in order to increase their access to oxygen [1].
Researchers led by Albert Libchaber of Rockefeller University in New York wanted to study the movement of these bacteria in a drop of water on a microscope slide. The researchers were amazed to see that, when the cells bumped into either the slide or the glass cover slip, they did not rebound. Instead, they continued to rotate roughly 10 times per second while pressing against the surface, like a propeller unable to move forward. Moreover, multiple cells became bound into regular, 2D hexagonal lattice structures of 10- 1000 cells, analogous to atomic crystals, complete with lattice vacancies and faceted edges. The team decided to document and model these structures, the first examples researchers have seen of living microorganisms forming a crystal.
To explain this phenomenon, the researchers calculated the water flow around the spinning bacteria. Each cell creates a vortex that sucks water in from the sides and pushes it away from the surface. This flow keeps the bacteria pressed to the surface and also draws them together, creating a hydrodynamic version of the electromagnetic attraction that binds atoms in an atomic crystal.
The team also modeled the rotation of the crystals, which is driven by the rotation of the individual cells. The rotational forces on a crystal are produced solely by the cells at the edges, as the multiple forces on interior cells cancel out. The researchers’ equations show that, as a crystal grows, the increasing rotational forces at its edges eventually cause it to “melt” (fall apart) when it grows beyond a critical size, although the team has not yet determined what that size is. In addition, the team found that crystals containing specific numbers of cells that can form very stable hexagons—such as 7, 19, and 37—are particularly stable, and adding or removing a single cell can cause an entire crystal to melt. Team member Alexander Petroff says the researchers are planning to investigate these curious dynamics, which “let crystals count how many cells they’re composed of.”
The team found that three characteristics of the cells allow the crystals to form: (1) strong fluid flow generated by each cell, (2) a propensity to congregate closely in regions with higher dissolved oxygen, and (3) spherical shape covered with flagella. However, it’s difficult to make good observations in mud, so the researchers don’t know whether the bacteria crystallize in their natural environment, or whether doing so provides any benefit, compared with disordered veils. “What we’re definitely seeing here is a bacterium that’s solving a very peculiar problem of getting nutrients fast enough,” says Petroff. The bacteria are “doing it by self-organizing into a new state of matter that has a lot of interesting properties we can’t wait to understand,” he says.
Timothy Pedley of the University of Cambridge in the UK says that work in his own lab on Volvox carteri colonies that orbit around each other arrived at the same fluid dynamical equation in 2009 [2]. He has not seen crystal formation before but was not surprised by it. However, Knut Drescher of the Max Planck Institute for Terrestrial Microbiology in Marburg, Germany, whose Ph.D. work included the 2009 research with Pedley, is more positive. He says the results represent an important new example of microswimmer collective behavior.
This research is published in Physical Review Letters
Note added (20 May 2015): After this article appeared, we learned of one other example [3] of living cells forming a crystal, however the conditions were very different.
–Tim Wogan
Tim Wogan is a freelance science writer in London.
References
- T. Fenchel and R. N. Glud, “Veil Architecture in a Sulphide-Oxidizing Bacterium Enhances Countercurrent Flux,” Nature 394, 367 (1998)
- K. Drescher, K. C. Leptos, I. Tuval, T. Ishikawa, T. J. Pedley, and R. E. Goldstein, “Dancing Volvox: Hydrodynamic Bound States of Swimming Algae,” Phys. Rev. Lett. 102, 168101 (2009)
- I. H. Riedel, “A Self-Organized Vortex Array of Hydrodynamically Entrained Sperm Cells,” Science 309, 300 (2005)