Cold Results from Fast Lasers
Observing, harnessing, or testing of quantum-mechanical phenomena is central to most modern physics experiments. Cold atoms make excellent testbeds to realize such effects. Large samples of atoms are routinely trapped and cooled down to microkelvin temperatures or below [1]. They are also easily manipulated by electric or magnetic fields. Yet when unperturbed they can retain their initial quantum state for seconds or more—a characteristic that is crucial for experiments requiring long observation times to detect minute quantum effects. The near-universal technique to create large samples of cold atoms is laser cooling, which has become simple enough to be incorporated into undergraduate laboratory curricula. Unfortunately, however, not all atomic species are amenable to laser cooling, and there are no viable alternatives to cool such species. A team led by Wes Campbell at the University of California, Los Angeles, has now demonstrated [2] a modification of laser cooling that promises to broaden the applicability of the technique to new species such as carbon, hydrogen, and other building blocks of organic life.
Applications using cold-atom ensembles include tests of the standard model of particle physics, quantum simulations of condensed-matter systems, precision sensing, and atomic clocks. Cold-atom settings have also been used to constrain theories of cosmic dark energy [3] and are a proposed platform for quantum computation. These applications all typically require trapping the particles and cooling their motion to the minimum allowed by quantum mechanics—the motional ground state. The trapping ensures that particles do not wander away during a measurement, while starting with all particles in the same lowest-energy quantum state typically makes measurements easy to perform and interpret.
The simplest form of traditional laser cooling proceeds as follows: laser beams from six different directions (usually propagating parallel and antiparallel to three orthogonal axes in space) are aligned to intersect, and the laser’s frequency is set slightly below the energy of a chosen electronically excited state. Because of this frequency detuning, particles in such a beam arrangement will preferentially absorb photons from the laser beam propagating opposite to their initial velocity. When a particle absorbs one such photon and is excited to the desired state, the photon’s momentum is transferred to the particle, reducing its velocity. The particle will quickly decay back to the ground state and emit another photon. But because this second photon is emitted in a random direction, over many cycles of absorption and emission the net effect is a decrease in the particle’s velocity. Since the particle’s momentum can be much greater than the momentum of a single photon, an apt analogy is slowing down a bowling ball by repeatedly hitting it with tennis balls. The addition of a magnetic field with a quadrupole structure and judicious choice of light polarization can allow particle confinement, in addition to cooling, in a magneto-optical trap.
Only certain elements have been laser cooled to date: all alkali metals, some alkaline earth metals, some transition metals (Cr, Fe, Ag, Zn, Cd, and Hg), some post-transition metals (Al, Ga, and In), a few lanthanides (Dy, Ho, Er, Tm, and Yb), and some noble gases. Notably absent are the building blocks of organic life, such as carbon, hydrogen, nitrogen, and oxygen. Although these elements compose 96% of the human body, chemical reactions involving these species are not yet well understood on a quantum-mechanical level. Researchers want to comprehend how the rules that govern chemical reactions for these and other species are different at ultracold temperatures [4]. For example, chemical reactions that would otherwise require a given activation energy may still occur at ultracold temperatures due to the effect of quantum tunneling. Other odd effects may be observed; for example, certain chemical reactions at ultracold temperatures can be turned on and off by applying an electric field. Although applications such as atom-by-atom assembly of designer biomolecules have been proposed, it remains to be seen how understanding and controlling behavior at such cold temperatures will be most beneficial with respect to the room-temperature dynamics of organic life.
However, laser cooling of hydrogen should have an immediate impact. As the Universe’s most abundant element, hydrogen at temperatures of tens of kelvin is central to most astrophysical processes, including the formation of stars and the evolution of interstellar gas clouds, so samples of cold hydrogen could serve as testbeds to study these processes. Moreover, should hydrogen be laser cooled, extension to antihydrogen would likely follow quickly, as the standard model of particle physics predicts identical electronic transition wavelengths for the two. Spectroscopy of trapped antihydrogen could elucidate topics ranging from the cosmic matter-antimatter asymmetry problem to charge, parity, and time-reversal symmetry, to the gravitational properties of antimatter.
A primary barrier to the laser cooling of certain species is generating sufficient light for the absorption and emission cycle to occur fast enough that incoming particles are slowed and caught in the trap before passing through the intersecting laser beams. The species carbon, hydrogen, nitrogen, and oxygen are all expected to require laser light at wavelengths shorter than approximately 200 nm, in the so-called vacuum ultraviolet (VUV) range between the ultraviolet and x-ray regimes. Generating sufficient VUV light with lasers traditionally employed for laser cooling, which emit photons continuously, is difficult [5]. The same task is much easier for lasers that emit photons in pulses. However, laser cooling with pulsed lasers is not standard, and only a few demonstrations exist [6–8].
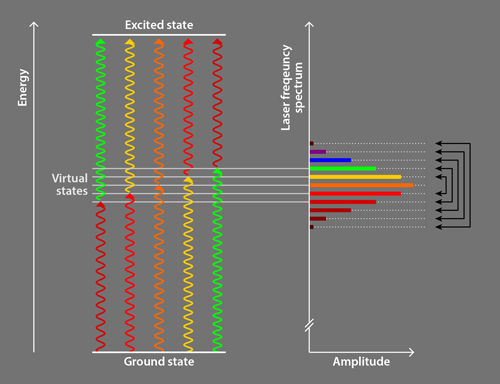
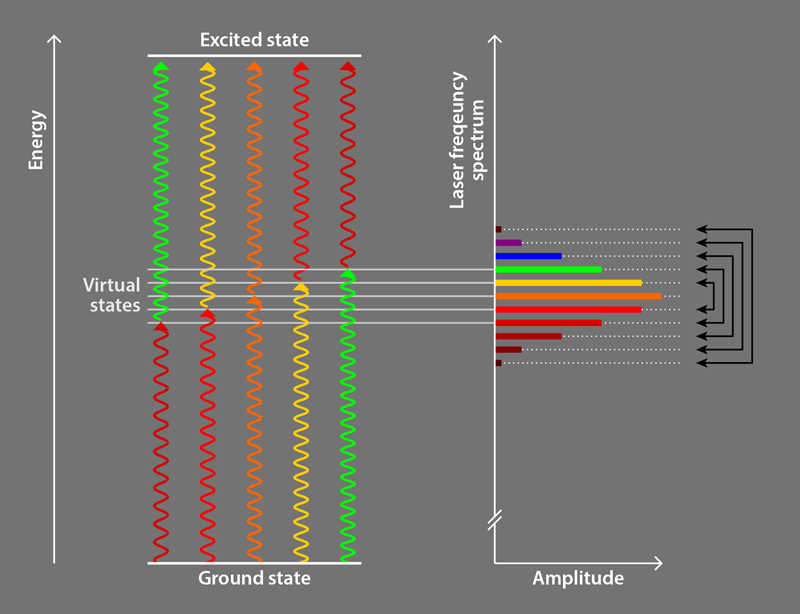
Following preliminary observations [9] and a theoretical proposal [10], the Campbell group [2] employed ultrafast pulses (lasting 2–5 ps) of laser light comprising a discrete set of evenly spaced frequencies. This light is commonly referred to as a frequency comb, and the discrete frequencies are called comb teeth. The researchers use a photon from one comb tooth to excite an atom into an intermediate virtual electronic state, and a photon from another comb tooth to excite it from the intermediate state to the desired excited state (Fig. 1). The comb teeth are paired so that all relevant photon pairs have the same sum energy, which is slightly less than the energy of the excited state; this energy difference ensures that particles preferentially absorb pairs of photons counterpropagating to the particles’ motion, in an analogous way to traditional laser cooling. All comb teeth are then used to drive the transition together [10], an innovative alternative compared to other groups’ approaches employing photons from a single comb tooth to excite atoms directly to the desired excited state [8]. The remaining aspects of the Campbell team method are similar to traditional methods.
Although the team utilized an ensemble of approximately 10 million rubidium atoms, a popular species for conventional laser cooling, the work’s implication is clear: frequency-comb cooling shows promise to laser cool species whose high transition energies rule out the use of continuous-wave lasers. Still, experimental challenges remain. While the final temperatures attained by the group are competitive with traditional laser-cooling methods, the photon absorption (and emission) rates realized are roughly 1000 times lower. Thus this approach will likely require a precooling method to catch initially hot particles, as the team employs for rubidium, before the frequency-comb cooling and trapping can begin. Given the immediate scientific interest in hydrogen (and antihydrogen), a challenging next step would be the demonstration of a viable precooling method for hydrogen compatible with the technique demonstrated here.
This research is published in Physical Review X.
References
- C. Salomon, J. Dalibard, W. D. Phillips, A. Clairon, and S. Guellati, “Laser Cooling of Cesium Atoms Below 3 K,” Europhys. Lett. 12, 683 (1990).
- A. M. Jayich, X. Long, and W. C. Campbell, “Direct Frequency Comb Laser Cooling and Trapping,” Phys. Rev. X 6, 041004 (2016).
- P. Hamilton, M. Jaffe, P. Haslinger, Q. Simmons, H. Müller, and J. Khoury, “Atom-Interferometry Constraints on Dark Energy,” Science 349, 849 (2015).
- B. K. Stuhl, M. T. Hummon, and J. Ye, “Cold State-Selected Molecular Collisions and Reactions,” Annu. Rev. Phys. Chem. 65, 501 (2014).
- K.-A. Brickman, M.-S. Chang, M. Acton, A. Chew, D. Matsukevich, P. C. Haljan, V. S. Bagnato, and C. Monroe, “Magneto-Optical Trapping of Cadmium,” Phys. Rev. A 76, 043411 (2007).
- I. D. Setija, H. G. C. Werij, O. J. Luiten, M. W. Reynolds, T. W. Hijmans, and J. T. M. Walraven, “Optical Cooling of Atomic Hydrogen in a Magnetic Trap,” Phys. Rev. Lett. 70, 2257 (1993).
- B. B. Blinov, R. N. Kohn, M. J. Madsen, P. Maunz, D. L. Moehring, and C. Monroe, “Broadband Laser Cooling of Trapped Atoms with Ultrafast Pulses,” J. Opt. Soc. Am. 23, 1170 (2006).
- J. Davila-Rodriguez, A. Ozawa, T. W. Hänsch, and T. Udem, “Doppler Cooling Trapped Ions with a UV Frequency Comb,” Phys. Rev. Lett. 116, 043002 (2016).
- A. Marian, M. C. Stowe, J. R. Lawall, D. Felinto, and J. Ye, “United Time-Frequency Spectroscopy for Dynamics and Global Structure,” Science 306, 2063 (2004).
- D. Kielpinski, “Laser Cooling of Atoms and Molecules with Ultrafast Pulses,” Phys. Rev. A 73, 063407 (2006).