Particles Stratify by Size in Thin Films
Paints and inks are typically colloids, consisting of tiny particles suspended in a liquid. As the liquid evaporates, the particles merge to form a thin film. Researchers have now demonstrated, both theoretically and experimentally, that particles of two different sizes, if they are large enough, separate out during drying, with the smaller particles forming a layer on top of the larger ones. By producing colloids that dry into a two-layer structure, the effect may make it possible to create cosmetics and electronic devices with novel properties.
As the liquid in a drying colloid evaporates, particles below the surface are forced closer together. At the same time, Brownian motion results in the particles jostling about randomly. Brownian motion is faster for smaller particles, so they can more easily redistribute themselves as the colloid volume decreases. Larger particles cannot move away so quickly and accumulate at the surface-air interface. Researchers who have studied colloids containing particles of different sizes have found that for certain evaporation rates, larger particles concentrated at the top of a drying film while smaller particles were more evenly distributed throughout [1]. It was widely assumed that the same was true at all evaporation rates.
Richard Sear of the University of Surrey in Guildford, UK, and his colleagues found exactly the opposite, however, when they examined a suspension containing two sizes of particles. “The stratification that we found was neither predicted nor expected, and we spent a few months verifying the results,” says team member Andrea Fortini, also of the University of Surrey.
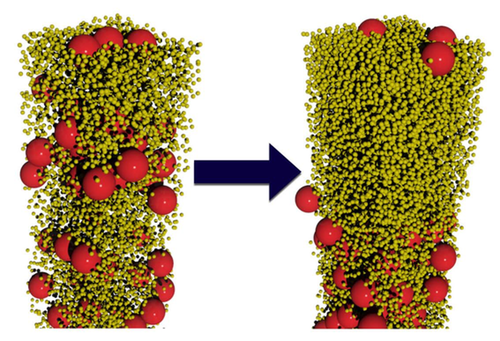
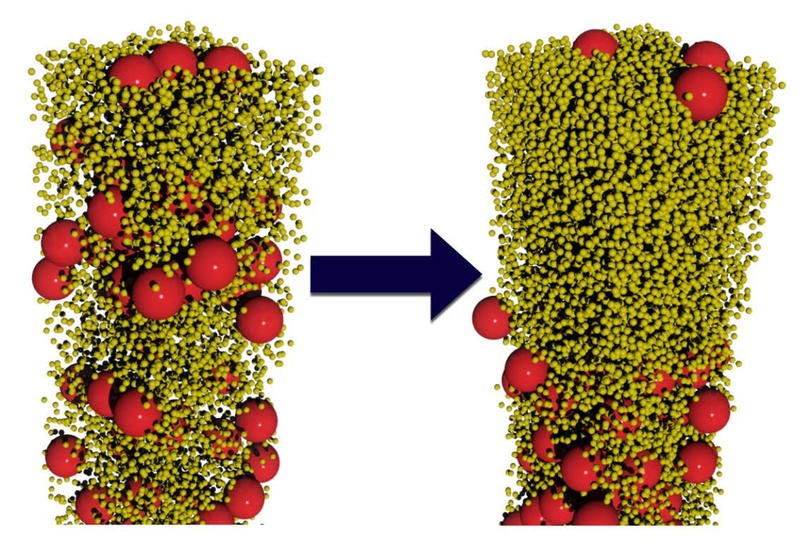
The researchers conducted computer simulations and lab experiments on water-based colloids containing spherical particles of two sizes. In the simulations, Fortini modeled suspensions containing large and small particles with various size ratios and with several different population ratios. As the water evaporated, the smaller spheres preferentially moved up while the larger spheres preferentially moved down. “Our simulations are scale independent; they can be applied to particles of any size in the colloidal regime,” says Fortini. This effect occurred for particle-size ratios ranging from 2:1 to 14:1, but only when the number of small particles exceeded the number of large ones by a factor of 200 or more. Even when stratification occurred, it was not perfect: some large particles remained trapped near the interface by surface tension.
The team experimentally verified the effect using acrylic particles with diameters of 385 and 55 nanometers suspended in water. They labeled the larger spheres with a red fluorescent dye. After allowing the colloids to dry, the researchers deduced the relative numbers of large and small particles throughout the layer by gauging its color intensity. Once again, they found that stratification spontaneously occurred, provided the numerical ratio of small to large particles was above 200.
The researchers explain their findings by imagining each particle as a ship’s sail, experiencing a force as other particles bounce off it. The net force is downward because there are more particles near the surface-air interface and fewer particles below. By accounting for this effect along with other details, the researchers deduced that the downward velocity of a particle is proportional to the square of its diameter. “Larger particles catch more ‘wind,’” Fortini says. Since the particle-size ratio in the experiments was 7:1, the larger particles moved down nearly 50 times faster than the smaller ones.
In previous studies, the researchers say, the smaller particles moved fast enough to maintain an approximately uniform distribution as the liquid evaporated. In the new work, however, the sizes of the small and large particles are such that both types move slowly, although at different rates, compared to the evaporation rate.
This stratification could pave the way for sunscreens with an ultraviolet-reflecting film over a moisturizing layer in contact with the skin. And in the electronics industry, a two-layer film with a scratch-resistant upper layer and a soft, sticky bottom layer would be useful for coating smartphone screens.
The study presents “a novel, yet simple, mechanism for the observed accumulation of small particles,” says Alexander Routh, a chemical engineering lecturer at the University of Cambridge. “It opens up a whole new avenue for how small particles will arrange themselves into structures.”
This research is published in Physical Review Letters.
–Katherine Kornei
Katherine Kornei is a freelance science writer in Portland, Oregon.
References
- R. E. Trueman, E. Lago Domingues, S. N. Emmett, M. W. Murray, and A. F. Routh, “Auto-Stratification in Drying Colloidal Dispersions: A Diffusive Model,” J. Colloid Interface Sci. 377, 207 (2012).