To Merge, Drops Must Separate
Mixtures as varied as salad dressings and crude oil can contain droplets of water in an oily solution. These droplets often merge continuously, which can lead to “unmixing.” Now researchers have watched individual pairs of droplets coalescing in a new way. As they report in the 18 January Physical Review Letters, the team was surprised to find that droplet fusion occurs as the pair is separating, rather than when they’re pushing against each other. The results may lead to a better understanding of the physics of unmixing in similar mixtures that are important in many industries.
Mixtures of two immiscible liquids, such as oil and water, are called emulsions. They are everywhere–in food products like mayonnaise and in the oil and pharmaceutical industries. Although people have been using emulsions for centuries, many of their behaviors remain somewhat mysterious. Coalescing droplets in emulsions involves phenomena spanning a range of scales, from the macroscopic to the microscopic, and previous studies have not been able to capture a complete picture of individual droplet collisions.
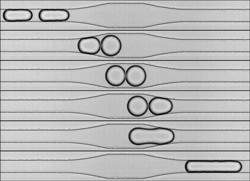
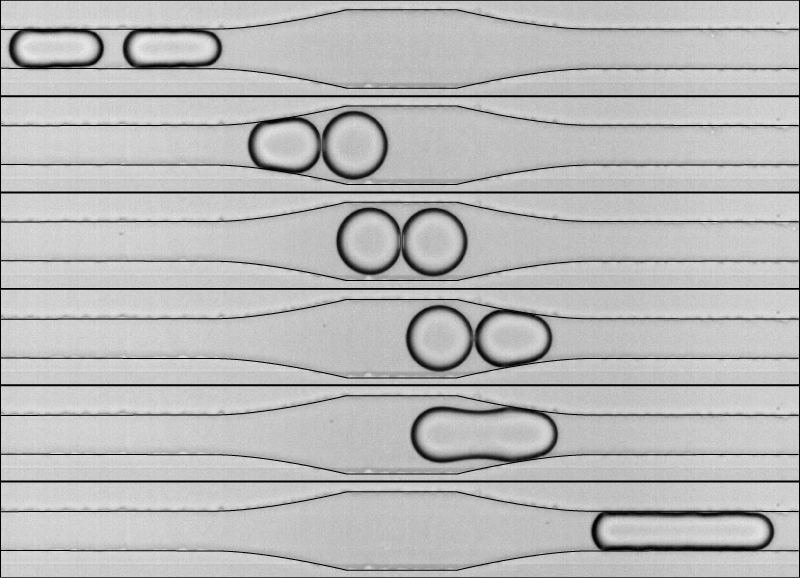
Now Nicolas Bremond, Abdou Thiam, and Jerome Bibette, from the Institute of Industrial Physics and Chemistry (ESPCI) in Paris, have developed a microfluidic device that allows controlled observation of thousands of individual pairs of coalescing water droplets. Their device shoots several hundred droplets per second–each just tens of microns in diameter–single file through a network of narrow channels filled with oil. The team created a moving train of droplet pairs, and each pair was forced to collide when it encountered a widening in the channel, because the wider channel suddenly slows the fluid flow. By measuring the changing distance between the droplets’ centers, the researchers found that mergers occurred moments after the droplets’ closest approach–when the droplets were separating.
To further study the coalescence, the researchers sent droplet pairs through a narrow channel that briefly widened and then became narrower again. The droplets collided when they hit the wider section, but then the leading droplet was sucked forward when it hit the constriction. As in the previous experiments, the mergers always occurred during the droplets’ separation phase. The team believes that the separation momentarily reduces the fluid pressure between the droplets, causing the higher-pressure water inside the droplets to burst through their adjacent barriers. Even when the team added a surfactant, a substance commonly used in industrial emulsions to prevent coalescence, they were still able to force droplets to merge. Since most emulsions used in industry contain surfactants, the results suggest that similar mergers can also happen in real-world situations.
The researchers also demonstrated that the mechanism could cause a chain reaction in a train of many closely-spaced droplets. After the leading pair merged, the rest followed quickly in sequence. Upon merging, the leading pair pulls away from the next droplet, triggering another merger as the effect propagates down the chain. According to the researchers, this run-away coalescence could be the principle behind the rapid clumping of droplets in industrial emulsions.
Furthermore, the team says the phenomenon they discovered could explain coalescence in all emulsions that are moving and flowing. Their results could open a new chapter in emulsion science textbooks, Bibette says, because no one has ever observed individually coalescing droplets in such a controlled environment.
The observations are indeed unique, agrees Thomas Mason of the University of California, Los Angeles. He thinks the chain reaction of colliding droplets is particularly exciting. This work, he says, can shed light on the basic physics behind some of the emulsion behavior long observed–and used–in industry, but that have never been fully understood.
–Marcus Woo
Marcus Woo is a freelance science writer in the San Francisco Bay Area.